Table 5.
Amination with Electron-Poor Substancesa

| |||
|---|---|---|---|
| entry | RNH2 | product | yield, % |
| 1 | TsNH2 |
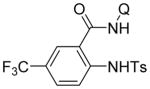
|
82 |
| 2 | 2-thiophene-SO2NH2 |
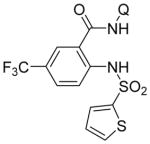
|
77 |
| 3 | 4-NO2C6H4-SO2NH2 |
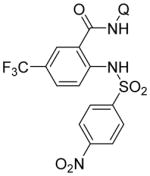
|
81 |
| 4 | 2-NO2-4-CF3C6H3NH2 |
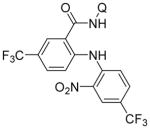
|
62 |
| 5 | 4-CF3C6F4NH2 |
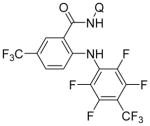
|
62 |
| 6 | 2-amino-5-chloropyridine |

|
48 |
Benzamide (1.0 mmol), (CuOH)2CO3 (0.5 mmol), pyridine (2.0 mL), amine (1.5 mmol), tetramethylguanidine (1.2 mmol) 5–8 h, 130 °C. Yields are isolated yields. Please see Supporting Information for details.
