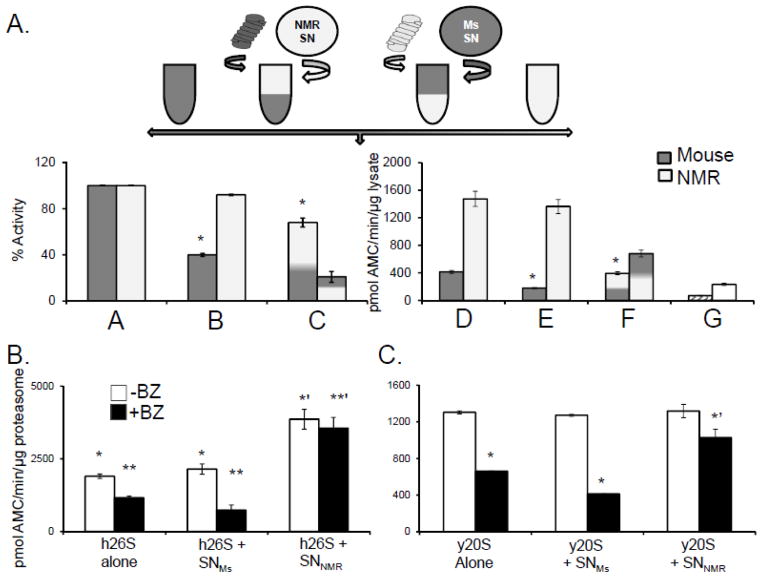
FIGURE 3. An NMR cytosolic factor confers resistance to mammalian and yeast proteasomes from competitive inhibition.
(A)Top panel: Schematic showing the design of the cross over experiment set up to evaluate if species differences in proteasome resistance to inhibition reflect an intrinsic property of the proteasome or the presence of a protective cytosolic factor. See Crossover Assays in Methods below for details. Bottom panel: When ProtMs were resuspended in SNNMR they showed elevated activity and inhibition resistance, whereas ProtNMR exposed to SNMS displayed both lower activity and greater sensitivity to inhibition. Bars A and D represents the activity of reconstituted MS and NMR cytosols (A = percentage set to 100% activity and D= ChT-L activity per mg lysate) in the absence of any inhibitor. B and E reveal the change in activity following treatment of the reconstituted cytosols with 20 μM MG132 and indicate that in comparison with mouse, the proteasome in NMR cytosol is resistant to inhibition. Bars C and F demonstrate that when ProtMs were resuspended in SNNMR they exhibited elevated activity and acquired inhibition resistance (p<0.05), whereas ProtNMR resuspended in SNMs showed both lower activity and greater sensitivity to inhibition (p<0.05) (means ± S.E.M.; n =5). Bar G reveals that the SN alone had very low peptidolytic activity. (B) Human 26S proteasomes [h26S] treated with SNNMR, but not SNMs or buffer, showed increased ChT-L activity (solid bars) and pronounced resistance to 10nM BZ (hatched bars) (* to *’, p<0.003; ** to **’, p<0.0004; means ± S.E.M; n = 6). (C) SNNMR conveyed inhibition resistance to the yeast proteasome [y20S]. SNMS did not show this effect. No significant increase in activity in the presence of SNNMR was detected. (* and ** indicated p < 0.01; means ± S.E.M.; n = 3)