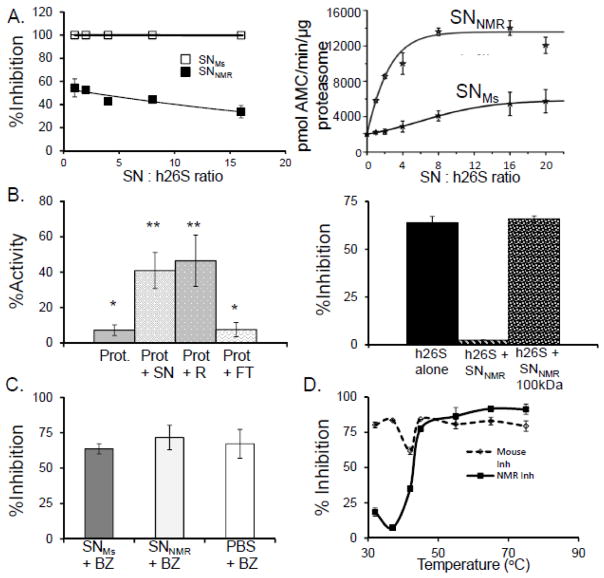
FIGURE 4. NMR cytosol contains a protein factor conveying resistance to inhibition and leading to activation of the proteasome.
(A) Titration of human 26S proteasome [h26S] with increasing protein concentration of mouse supernatant [SNMS] (open squares) did not result in acquiring resistance to inhibition with 20 μM MG132 even at 16-fold excess of SNMS. In contrast, resistance was evident even at lowest used SNNMR: h26S ratio (1:1, 250ng of each component), and was slightly stronger with increasing SNNMR: h26S ratio (left panel; closed squares). Chymotrypsin-like [ChT-L] activity of h26S was rapidly and profoundly enhanced by the addition of SNNMR, reaching a plateau with more than 6-fold activation at about 8:1 (SNNMR: h26S) ratio (right panel). However, treatment with the 8-fold excess of SNMS resulted in only 2-fold activation of h26S, and the activation never exceeded 3-fold under the conditions used (n =5). (B) SNNMR was fractionated with a spin filter with 3,000 Da pore cutoff membrane (top panel) or with a 100,000 Da pore cutoff membrane (bottom panel). Top panel demonstrates ChT-L activities of ProtNMR after treatment with 20 μM MG132, alone or with addition of the whole SNNMR, the 3,000 Da retentate (RNMR; molecules with apparent molecular weights higher than 3,000 Da) or the 3,000 Da filtrate [FTNMR; molecules with apparent molecular weights lower than 3,000 Da]. The activity of inhibitor-challenged ProtNMR was markedly higher after treatment with the whole SNNMR (compare with Fig. 2A, bottom panel) or with the RNMR, but not with FTNMR. Spin-filtering the SNNMR through a 100,000 Da pore cutoff membrane, and performing the ChT-L activity assay with h26S treated with 20μM of MG132 (left bar) the inhibition resistance was conveyed by the SNNMR (middle bar; compare with Fig. 2B) but not by the filtrate (right bar; MW>100,000 Da, n =5 each treatment). (C) The ChT-L activity of h26S proteasome was markedly inhibited by all three filtrates, indicating that BZ was not significantly sequestered by SNNMR, as compared with SNMs or buffer (n = 6). (D) To test if the factor is heat liable we subjected SNNMR and SNMs to heat stress at temperatures ranging from 32°C to 75°C. The heat-treated SNs were then added to h26S and ChT-L proteasome activity and inhibition resistance were assessed with 10nM BZ. Heat-treatment above 45°C ablated the inhibition resistance conveyed by SNNMR strongly suggesting a protein nature of the proteasome-affecting factor (n = 5).