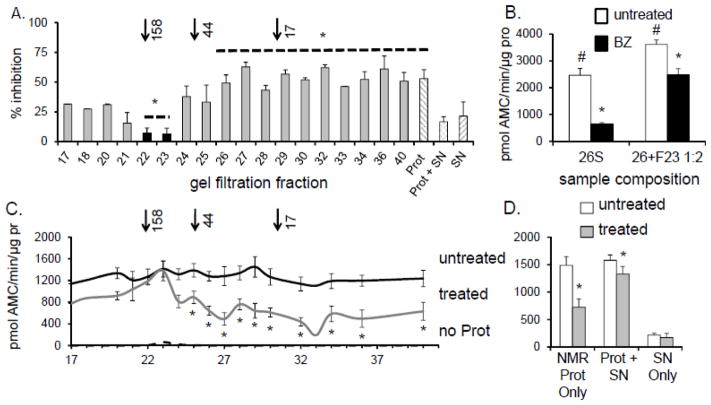
FIGURE 5. NMR proteasome resistance to inhibition was found in two gel filtration fractions.
Proteasome-depleted SNNMR was fractionated by gel filtration. (A) ProtNMR was added to the gel filtration fractions and proteasome-specific inhibition resistance was measured after treatment of each fraction with 20 μM MG132. Only fractions 22 and 23 consistently protected the ProtNMR ChT-L activity from MG132 inhibition (*p<0.05; mean ± S.E.M., n = 5). Although fr.21 contained HSP72 and HSP 40 it did not confer significant inhibition resistance. Arrows above A indicate elution of the molecular weight standards. (B) ChT-L activity (expressed per mg protein, [pro]) of human 26S proteasome [h26S] was significantly increased and protected from inhibition by 10 nM BZ when suspended in the resistance factor-enriched fr.23 (#p<0.01, *p<0.0004). Treatment with 10 nM BZ ablated activity of h26S by 75%. In the presence of fr. 23 ChT-L activity of h26S exposed to BZ was reduced only by 30%. (C) Naked mole-rat [NMR] proteasome-enriched pellet [ProtNMR] was added to the gel filtration fractions and chymotrypsin-like [ChT-L] activity was measured after treatment with 20μM, MG132 (“treated”) or DMSO vehicle (“untreated”). Essentially no ChT-L activity was detected in the fractions in the absence of ProtNMR (“no Prot”). (*p<0.05; mean ± S.E.M.; n = 5). Only ProtNMR added to fractions 22 and 23 was refractory to MG132. (D) ProtNMR alone was sensitive to MG132 inhibition, unlike ProtNMR re-suspended in SNNMR (*p < 0.02). Very low peptidolytic activity was detected in SNNMR (see also C, hashed columns).