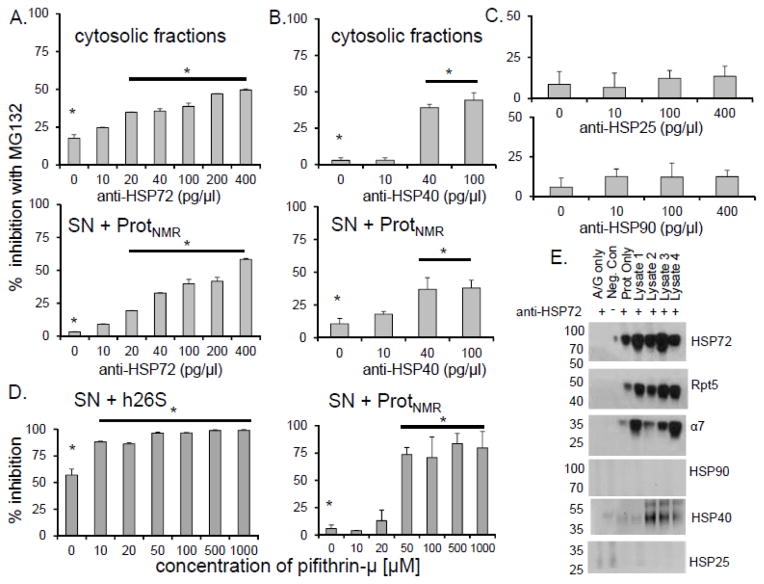
FIGURE 7. HSP72 and HSP40 played a critical role in inhibition resistance of naked mole-rat proteasomes.
(A, B) Titration of the naked mole-rat cytosolic fraction and SNNMR with increasing amounts of neutralizing antibodies against HSP72 and HSP40 significantly decreased the level of inhibition resistance (*p<0.001) (mean ± S.E.M., n = 6). (C) Anti-HSP25 or anti-HSP90 did not affect sensitivity to inhibition. (D) Treatment with increasing concentrations of the HSP72-specific inhibitor pifithrin-μ attenuated resistance to inhibition (*p<0.01) (mean ± S.E.M., n = 5). (E) Immunoprecipitation with anti-HSP70/72 antibody showed interaction between HSP72 and the NMR 26S (α7, Rpt5) as well as with HSP40, but not with HSP25 or HSP90.