Abstract
The human menopause transition and aging are each associated with an increase in a variety of health risk factors including, but not limited to, cardiovascular disease, osteoporosis, cancer, diabetes, stroke, sexual dysfunction, affective disorders, sleep disturbances, and cognitive decline. It is often challenging to systematically evaluate the biological underpinnings associated with the menopause transition in the human population. For this reason, rodent models have been invaluable tools for studying the impact of gonadal hormone fluctuations and eventual decline on a variety of body systems. While it is essential to keep in mind that some of the mechanisms associated with aging and the transition into a reproductively senescent state can differ when translating from one species to another, animal models provide researchers with opportunities to gain a fundamental understanding of the key elements underlying reproduction and aging processes, paving the way to explore novel pathways for intervention associated with known health risks. Here, we discuss the utility of several rodent models used in the laboratory for translational menopause research, examining the benefits and drawbacks in helping us to better understand aging and the menopause transition in women. The rodent models discussed are ovary-intact, ovariectomy, and 4-vinylcylohexene diepoxide for the menopause transition. We then describe how these models may be implemented in the laboratory, particularly in the context of cognition. Ultimately, we aim to use these animal models to elucidate novel perspectives and interventions for maintaining a high quality of life in women, and to potentially prevent or postpone the onset of negative health consequences associated with these significant life changes during aging.
Keywords: Aging, Menopause, Rodent, Model, Hormones, Female
Introduction
Natural aging processes result in physiological changes in the brain and body of all organisms. In most mammalian species, females experience natural reproductive senescence in mid- to late- life. While the vast majority of species’ lifespans do not far surpass their reproductive years, one clear exception to this common phenomenon is the human female, whose lifespan extends well beyond the reproductive life stage. For women, reproductive senescence typically occurs around the fifth decade of life, when their finite pool of immature ovarian follicles is depleted via a combination of ovulatory cycles and normal apoptosis (i.e., programmed cell death) called atresia. Menopause ensues when the menstrual cycle ceases due to anovulation, and is verified retrospectively after one year of amenorrhea [1–2]. Notably, menopause is not an abrupt event; indeed, the transition to menopause typically spans four to six years [3]. The onset of the menopause transition and subsequent post-reproductive life stage comes with a variety of physiological, behavioral, and brain changes that can impact quality of life [4–6]. Many health risk factors change with aging and after menopause, including, but not limited to, an increased risk for cardiovascular disease, osteoporosis, cancer, weight gain, diabetes, stroke, sexual dysfunction, affective disorders, sleep disturbances, and cognitive decline [1, 6–7].
Currently, the estimated average female lifespan at birth worldwide is 70 years [8], and life expectancy is increasing, especially for women [9]. Given these numbers, the post-reproductive life stage now encompasses about a third of a woman’s lifespan on average. Presently, about 14.88% of United States (U.S.) residents are over the age of 65 (of which about 56% are female) [8]. By 2030, as the “baby boomer” generation ages and life expectancy continues to increase, approximately one out of five people will be aged 65 and older in the U.S. [10–11]. With over 6,000 women projected to reach menopause each day in the U.S. alone [1], it is imperative that we understand the impact of natural and surgical gonadal hormone loss as women reach the post-reproductive life stage in order to inform researchers and clinicians about options for women to maintain a high quality of life across the entire lifespan.
Remarkable work has been accomplished in the realm of clinical research on women’s health. Yet, the biological underpinnings of the menopause transition can be challenging to systematically evaluate in humans due to heterogeneous variations in many life factors, including age, parity, diet, socioeconomic status, environmental exposures, and genetic characteristics. This rich variability in humans can make it difficult to dissociate genetic and environmental factors while evaluating specific variables associated with aging and menopause. As such, animal models have been instrumental in evaluating gonadal hormone effects on various body systems, including developmental and cognitive processes, while also providing a more homogeneous population to study in the laboratory compared to humans [12]. There are several well-characterized laboratory animal models used to evaluate the effects of gonadal hormones and aging, including non-human primates and rodents. Non-human primate models are especially insightful for translational research because of their genetic, physiological, behavioral, and reproductive system similarities to humans [13–16]; however, they are costly and often require many years of study to elucidate the impact of aging and reproductive senescence due to their increased longevity relative to rodents. Rodent models, especially laboratory rats and mice, are particularly useful because of their well-defined aging trajectories and thoroughly studied brain and reproductive systems, as well as their approximate two to three year lifespan [17]. This review focuses on these rodent models, comparing and contrasting the benefits and drawbacks of rodent models of reproductive senescence, and discussing the utility of the rodent model for translational menopause and aging research.
1.1 The Human Menopause Transition
It is a well-accepted tenet that women are born with a finite pool of immature ovarian follicles. Histological follicle counts and mathematical models of ovarian follicle reserves estimate an average of 295,000 follicles per ovary at birth to 180,000 follicles per ovary at puberty; as the menopause transition approaches, estimates are reduced to between 100–1000 immature follicles per ovary [18–19]. At puberty, the ovulatory cycle begins as a result of the maturation of the hypothalamic-pituitary-gonadal (HPG) feedback loop. Women experience regular ovulation, and therefore a consistent menstrual cycle, for about 40 years. Approximately 400 eggs are ovulated across a woman’s reproductive life stage; the remainder — and vast majority — of ovarian follicles undergo atresia [20–21].
The female reproductive cycle involves an intricate feedback system between the brain, pituitary gland, and reproductive tract. The hypothalamus synthesizes and releases gonadotropin releasing hormone (GnRH) from GnRH neurons. GnRH signals the anterior pituitary gland to synthesize and secrete the gonadotropins follicle stimulating hormone (FSH) and luteinizing hormone (LH) into the general bloodstream. Immature ovarian follicles undergo various developmental stages over the course of several months and are classified by size and the different cell types that comprise the growing follicle (Figure 1) [18, 22]. Once the follicle pool naturally depletes over time, menopause ensues. What initiates the transition to menopause, whether it is the declining follicle pool and/or brain changes resulting in dysregulation in the HPG axis, is a topic of debate. Phyllis Wise’s elegant work in rodents and non-human primates suggests that the central nervous system is of chief importance for the onset of reproductive senescence and that, ultimately, a breakdown in communication between the brain and ovaries results in anovulatory cycles and eventual cessation of the menstrual cycle. This landmark research, discussed in section 3.1.1, suggests that a complex set of changes in neuroendocrine and neurotransmitter signaling involving hypothalamic GnRH neurons and alterations in glutamatergic, GABAergic, and monoaminergic signaling, likely play roles in early stages of the transition to a reproductively senescent state for rodents, non-human primates, and women alike [23–28].
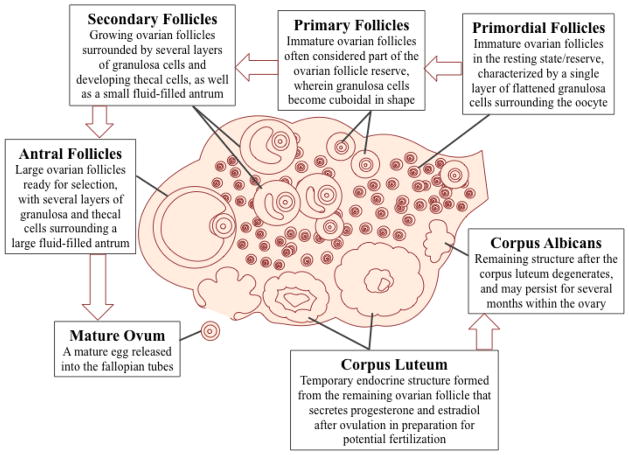
Figure 1.
Stages of Ovarian Follicle Development Stages of ovarian follicle development, beginning at the primordial resting stage and growing in size and shape through the antral (pre-ovulatory) mature follicle. Once the ovum is ovulated, the remaining follicle forms the corpus luteum, a temporary endocrine structure that produces high amounts of progesterone and some estradiol. The corpus luteum degenerates into the corpus albicans if no egg fertilization occurs.
Around the onset of the menopause transition, women may opt to take hormone therapy (HT) to attenuate some of the negative physiological symptoms associated with menopause, such as vasomotor and genitourinary symptoms [29]. Estrogens have been shown to have beneficial effects on a myriad of body systems, including cardiovascular, bone, and brain health. However, estrogens’ actions are complex, and often exert beneficial effects only when given at a fitting dose, through the ideal route of administration, and with appropriate timing and duration of the treatment [30–32]. In the early 2000’s, findings from the Women’s Health Initiative Memory Study (WHIMS) challenged the tenet that estrogen-containing HT has protective effects against global cognitive decline, mild cognitive impairment, and probable dementia risks when the large, double-blind, placebo-controlled clinical trials indicated that some forms of HT increase the risk of probable dementia development when administered post-menopause [33–36]. The WHIMS findings propelled the field forward; the window of opportunity and other hypotheses for therapeutic benefits of estrogen-including HT arose, with a particular emphasis on cognitive aging [37–41]. Specifically, the window of opportunity is thought to be a critical period, likely during the early stages of the menopause transition wherein estrogenic HT may confer a benefit to memory systems and delay the onset of cognitive decline or dementia [42]. Thus, the field is focused on furthering understanding of the menopause transition to help identify a critical window for therapeutic benefits and possible intervention to lower risk factors associated with aging and menopause.
Of note, the window of opportunity is likely not a singular factor, but could be thought of as a profile that encompasses several constitutive elements associated with aging and menopause, such as the age at which a woman begins to transition, the length of time since the impetus of the transition, exposures to both exogenous hormone administration (e.g., oral contraceptives) and transient endogenous changes in ovarian hormone milieu (e.g., pregnancy), as well as maternal family history of menopause length and symptomology, and gynecological surgical interventions. This broad spectrum of factors is often difficult to assess in the healthy aging woman; indeed, much of our insight into human factors comes from studying disease states, which may be a confounding factor itself. We have turned to rodent models to systematically explore brain and behavioral effects associated with timing in this critical window [43–47]. These models can teach us the optimal parameters for timing, duration, dose, formulation, and routes of administration by allowing direct manipulation, while also permitting control over factors such as parity and previous exogenous hormone exposures.
2.1 Rodent Aging and Reproductive Senescence
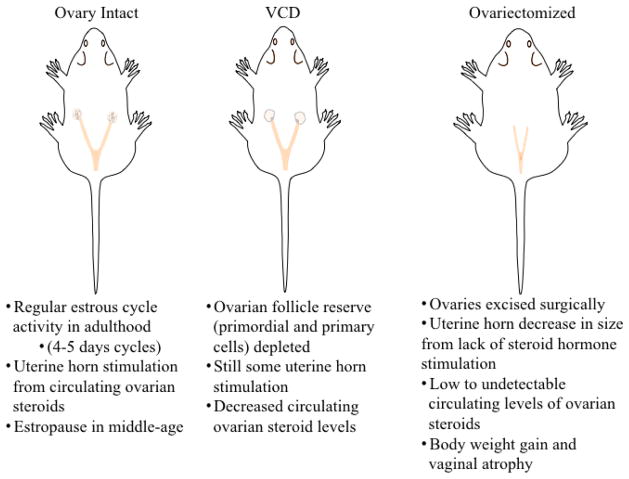
Before we can fully appreciate rodents as valuable and appropriate models for menopause and aging research [48–49], we must first acknowledge some key differences between human and rodent reproductive senescence [23, 50]. Rodents have an estrous cycle rather than a menstrual cycle. The rodent estrous cycle differs in several ways, including that rodents’ uterine lining is reabsorbed rather than shed via menstruation. The estrous cycle occurs every four to five days and consists of four phases — proestrus, estrus, metestrus, and diestrus — that involve similar ovarian hormone fluctuations to the human menstrual cycle (Figure 2A,B) [51–52]. Around 9–12 months of age, rats and mice typically experience irregular estrous cycles, aptly termed estropause. A constant/persistent estrus state is common, wherein the estrus phase is prolonged and anovulatory cycles may occur (Figure 3A). Animals may then transition into an anestrous state, where ovulatory cycles halt and low levels of gonadal steroids are present [53–55]. Rats, more often than mice, will also pass through a phase of repetitive pseudopregnancy prior to transitioning into an anestrous state, or they may stay in a pseudopregnant state for their remaining lifespan, wherein they ovulate irregularly (and sometimes supraovulate), resulting in corpora lutea that are maintained for an extended period of time producing high progesterone levels [50, 55]. Rodents also experience dysregulated HPG axis activity in estropause, including decreased LH release responsiveness to estrogen signaling in middle-aged rats [56–57]. A chief difference between humans and rodents is the presence of potentially mature ovulatory follicles in the aged rodent. Rodents experience some natural ovarian follicular depletion and are also suspected to have a finite follicle pool [58]; however, some preliminary evidence for adult neo-oogenesis in rodents has challenged this idea [59–60]. Because a hallmark of human menopause is complete ovarian failure, it is important to keep these differences in mind when choosing an appropriate animal model of menopause. Several rodent models of reproductive aging are implemented in the laboratory to aid in understanding of brain and body aging and the menopause transition, considered in detail below.
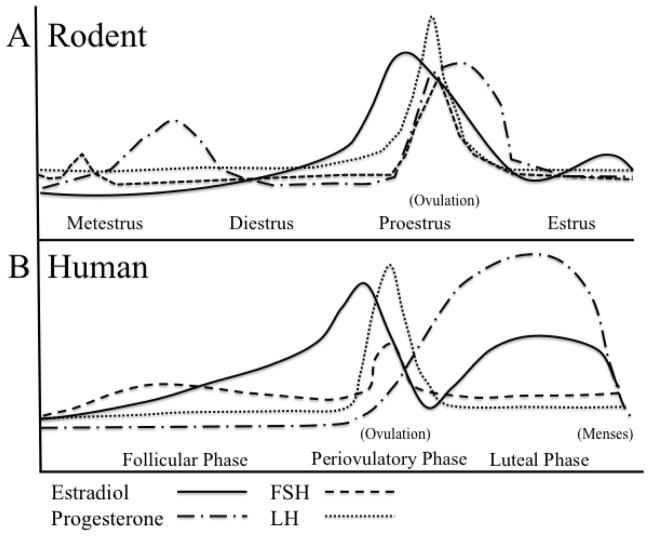
Figure 2.
The Ovarian Hormone Cycle During the Reproductive Stage A) The rodent estrous cycle is characterized by four phases. Proestrus is the shortest phase, lasting less than a day, wherein a critical level of increasing 17β-estradiol is thought to trigger the LH surge, inducing ovulation. FSH and progesterone also peak during proestrus. The proestrus phase is followed by the estrus phase. In estrus, LH, FSH, and progesterone decline to baseline levels, while 17β-estradiol levels are moderately low. In metestrus, 17β-estradiol is low, and a transient increase in FSH occurs. Some 17β-estradiol and high amounts of progesterone are released from the corpora lutea following ovulation, increasing circulating progesterone levels between the metestrus and diestrus phases. Gonadal hormones return to baseline as the estrous cycle begins again. B) The human menstrual cycle has three distinct phases. The follicular phase is characterized by steadily increasing 17β-estradiol levels. At a critical level of 17β-estradiol, LH surges, with the trigger for ovulation occurring in the periovulatory phase. Ovulation is followed by the luteal phase, wherein progesterone levels increase and 17β-estradiol is present at a moderate level.
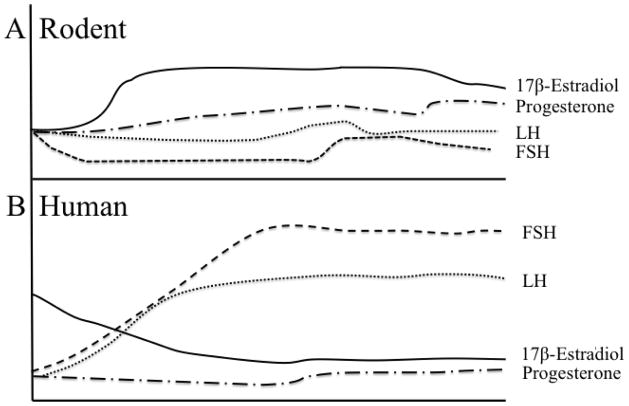
Figure 3.
Ovarian Hormone Levels in Reproductive SenescenceA) Rodents commonly enter a persistent estrus state during reproductive senescence (estropause), characterized by moderate to high 17β-estradiol levels and moderate progesterone, LH, and FSH levels as a result of disrupted feedback between the ovaries and hypothalamus/pituitary. b) Human reproductive senescence (menopause) is characterized by low, sometimes undetectable, levels of 17β-estradiol and progesterone, but increased levels of the gonadotropins, FSH and LH.
3.1 Rodent Models of Menopause
3.1.1 Ovary-intact: A model of the aging hypothalamic-pituitary-gonadal axis
Similarly to women, rodents experience regular reproductive cycles in adulthood, as well as age-related dysregulation of this cycle and the HPG axis, ovarian changes, and gonadal hormone fluctuations. Many researchers have utilized the ovary-intact female rat as a model of human menopause to evaluate the changing brain, pituitary, and ovary interactions with age [23–24, 61]. Phyllis Wise’s research, beginning in the 1970’s, demonstrated that as aging occurs in ovary-intact rats, changes occur in the rhythmicity of hypothalamic GnRH release that initiates the LH surge. These alterations, originating in the brain, precede observable changes in normal estrous cyclicity in aging rodents [62–63]. These alterations are particularly evident during the proestrus phase, wherein a LH and FSH surge precedes ovulation. Wise’s laboratory found that middle-aged animals had lower pre-ovulatory circulating estradiol levels, and that before the LH surge, there was a temporal delay in rising GnRH concentrations in the median eminence, which connects the hypothalamus to the pituitary gland and facilitates gonadotropin release via the hypophysial portal blood stream; decreased serum LH, estradiol, and progesterone levels following the LH surge in middle-aged animals point to an age-related disruption in circadian rhythm function that consequently alters normal estrous cycle patterns [62]. Wise and others have also found that the amplitude of the LH, FSH, and prolactin surges was reduced in middle-aged, ovary-intact animals [63–64]. These landmark studies gave rise to the theory of aging of neural pacemakers, which suggests both chronological and endocrine aging are crucial factors for understanding the transition to reproductive senescence, and that the brain plays a significant role in these processes. Using the ovary-intact rat model, the Gore laboratory has evaluated aging and its relationship to neuroendocrine control and feedback mechanisms. This team found increased GnRH mRNA levels in middle-aged and aged rats compared to young control subjects; yet, gene transcription for GnRH decreased with age, pointing to a potential posttranscriptional mechanism for the age-related increase in GnRH mRNA [65]. GnRH and LH release are, in part, mediated by excitatory glutamate signaling via input from N-methyl-D-aspartate (NMDA) receptors; alterations in these inputs likely disrupt positive and negative feedback mechanisms of the HPG axis regulation [65]. Although the number of GnRH neurons does not appear to change with age, there are age- and reproductive senescence- related changes in the gene expression of the subunits that constitute the NMDA receptor [65–66]. Gore and others have suggested that during aging, there is attenuated NMDA receptor-mediated glutamatergic activation of GnRH-producing neurons in brain regions important for HPG feedback [65, 67–68]. This disrupted balance between excitatory and inhibitory neurotransmission is related to the transition to reproductive senescence [61]. These alterations in neuroendocrine function may occur prior to animals displaying outward behavioral or estrous cycle abnormalities, and would likely be difficult to target studying human clinical populations. Therefore, utilizing the ovary-intact rat model to evaluate the natural age-related cellular and molecular changes in brain regions involved in normal reproductive functioning and feedback is crucial to understanding neurological changes that occur in women, and to the development of novel therapeutic targets to mitigate negative symptoms associated with age and reproductive senescence.
A drawback to modeling menopause in the ovary-intact rodent is the difference in the ovarian follicle reserve and steroid hormone changes as aging ensues in the rat compared to women. Because rodents do not typically exhibit follicle-deplete ovaries by the onset of reproductive senescence like women do, circulating gonadal hormone levels in an aged ovary-intact rodent do not closely resemble those of menopausal women. Women in the post-menopausal state have very low circulating levels of 17β-estradiol and progesterone and significantly elevated FSH and LH levels (Figure 3B) [52, 69]. Rodents, on the other hand, experience several reproductive-aging states and typically maintain moderate circulating 17β-estradiol levels, despite HPG axis dysregulation [55] (discussed in 2.1). Variations in the onset and expression of HPG axis changes in the ovary-intact female rat could add undesirable variability to this menopause model; however, as we gain understanding of these changes, and if we acknowledge these variations when interpreting findings, we can use this natural variation to our experimental advantage. Vaginal cytology monitoring is a common, minimally invasive way to monitor estrous cycle activity (Figure 4). Yet, vaginal cytology can become erratic in middle age due to dysregulated HPG axis activity. If a researcher intends to use a particular hormone profile of a middle-aged rodent, such as constant estrous, for an experiment, it is likely that only a subset of animals in a colony will fall under this profile at the appropriate age and timing for the experiment. While studying the natural diversity that occurs in the ovary-intact model and taking these estrous cycle variations into account benefits correlation data evaluations, it is limiting in causal-type inferences. It is preferable to methodically control reproductive status and age at evaluation in experimental animals in order to dissociate the specific effects of reproductive senescence from advanced aging, since confounding factors due to age alone may muddle results.
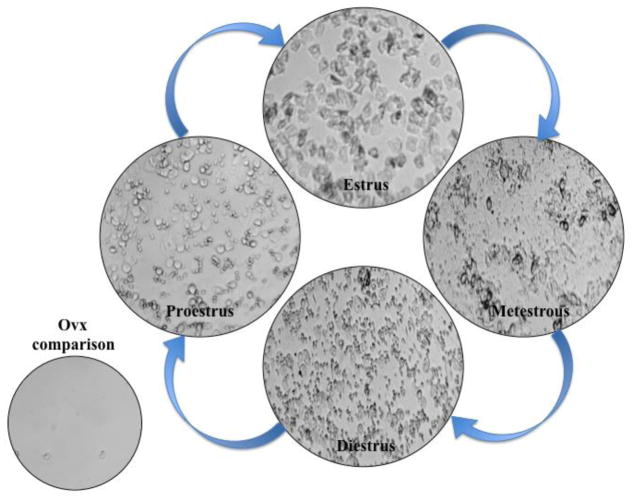
Figure 4.
The Estrous Cycle Representative images of the rat estrous cycle with vaginal cytology. Animals in estrus exhibit cornified cells in their vaginal smears. Metestrus smears contain a combination of cornified cells, leukocytes, needle-like, and round epithelial cells. Diestrus smears are characterized by a large number of leukocytes, and proestrus smears contain round epithelial cells and some cornified cells, sometimes presenting in clustered groups.
Some stocks or strains of laboratory rodents are considered better models than others for aging research. One example is the National Institute on Aging’s (NIA) recommendation to use the Fischer-344 (F344) inbred strain for aging research due to their longevity and well-defined aging patterns. Sprague Dawley and Long Evans are also commonly utilized rat strains in aging research. Most laboratory rodent colonies do not have aging animals in stock; those that are available are often limited to retired breeders. In menopause research, reproductive history is a variable of chief importance – indeed, several laboratories have demonstrated that sexual experience and parity influence cognitive and brain outcomes [70–74]. For example, these studies suggest a history of pregnancy is related to enhanced spatial memory performance compared to nulliparous animals, and brain responsiveness to estrogen treatment may be influenced by reproductive history. Thus, when employing the ovary-intact rodent model for aging and menopause work, scientists must be carefully consider the stock or strain of rodent as well as their age and life history to obtain an appropriate aging model for their research questions regarding the transition to reproductive senescence.
3.1.2 Ovariectomy: A model of surgical menopause
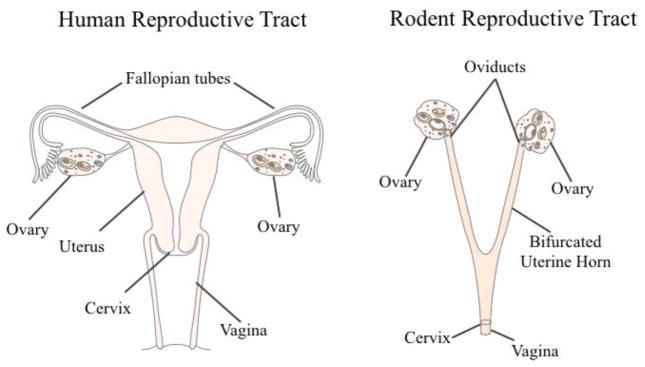
The gold standard in the preclinical field for evaluating gonadal hormone effects in female animal models is the ovariectomy, or surgical removal of the ovaries, often abbreviated Ovx. Rodent anatomy differs from women in that rodents have a bifurcated uterus, called the uterine horn, which accommodates large litters (Figure 5). The standard Ovx procedure is to bilaterally excise the ovaries, oviducts (i.e., the fallopian tubes), and tips of the uterine horn from the peritoneal cavity, leaving the ligated uterine horns intact [75]. Full recovery from Ovx occurs within one week. Ovx most accurately models surgical menopause in women. Ovx is an ideal model to evaluate specific effects of gonadal hormone deprivation and subsequent exogenous hormone treatments on the brain and periphery because it creates a “blank gonadal hormone slate.” As an example, estrogens may be administered after Ovx, alone or in combination with other ovarian hormones, with variations in the type of estrogen administered, dose, route of administration, and treatment duration while controlling for interactions with endogenous levels of sex steroid hormones. Combined exogenous therapies, such as 17β-estradiol plus progesterone, may be used to evaluate their interactions on a particular system, more closely modeling HT regimens that many menopausal women take. Our laboratory and many others have utilized the Ovx model to evaluate the potential for neuroprotective effects of exogenous ovarian hormone administration on the brain. For example, administering HT to Ovx females has been shown to enhance performance on mazes evaluating spatial memory compared to Ovx females that were not receiving estrogen treatment (discussed in section 4.1). Higher circulating 17β-estradiol levels have been associated with better spatial memory performance for both young and middle-aged Ovx rats [47], and chronic, long-term exogenous estrogen plus progesterone treatment enhances radial-arm maze performance in middle age [45]. Importantly, the Ovx model has also shown limitations of efficacy in gonadal hormones, including some estrogens. For example, when estrone, a weaker metabolite of 17β-estradiol and the main component in Premarin, was given at a tonic dose to middle-aged Ovx rats, it had a negative impact on spatial memory at a high dose [76].
Figure 5.
Human and Rodent Reproductive Tracts A comparison of the human and rodent reproductive tracts, with analogous structures labeled.
A drawback to the Ovx model in the context of translational research is that the majority of women retain their whole reproductive tract throughout the menopause transition. While removing the ovaries prior to the onset of reproductive senescence allows researchers the control to evaluate the impact of particular hormones without aging as a confounding factor, the age at which Ovx occurs in rodents may also result in divergent effects [77–78], particularly for cognition [44]. Additionally, the sudden loss of ovarian steroids is not characteristic of the majority of transitionally menopausal women; compounded with this, the post-menopausal ovary continues to release androgens and low levels of other steroids [79–80]. Consequently, the Ovx model results in a considerably different hormone profile, as well as an altered HPG axis, compared to animals with an intact reproductive tract. Nonetheless, the Ovx model is a classic technique that merits much praise for its utility in evaluating novel phenomena in the context of aging and menopause research. While it is imperative to understand the impact of specific hormone and drug effects on the brain and body systems with a “blank gonadal hormone slate,” in the context of the menopause transition, these factors associated with reproductive senescence do not operate in a vacuum, and understanding how exogenous hormone formulations interact with intact systems (i.e., a more representative model of human menopause) is translationally important.
3.1.3 4-vinylcyclohexene diepoxide: A model of transitional menopause
The drug 4-vinylcyclohexene diepoxide, or VCD, a metabolite of 4-vinylcyclohexene, is produced when the industrial chemical butadiene is utilized to manufacture various plastic-based goods [81–82]. VCD is used commercially to reduce fecundity and species proliferation of rodent pests without the use of hazardous poisons that endanger the environment [83]. In the early 2000’s, VCD was introduced to biobehavioral animal research as a rodent model of transitional menopause because it was found to selectively target and deplete the non-growing ovarian follicle pool in rodents via atresia, resulting in accelerated follicular depletion and eventual ovarian failure in rodents [79–81, 84–93]. VCD is thought to function through accelerating atresia in primordial and primary ovarian cells — but not disrupting growing follicles — by altering the expression and distribution of the Bcl-2 family of proteins that regulate apoptosis, including Bcl-xL, Bax, and Bak proteins within ovarian follicles, thus initiating cytochrome c release into the cell cytosol, triggering downstream caspase signaling activity, all of which are related to accelerated follicle atresia [49, 84–85, 91–93].
The introduction of VCD as a model of transitional menopause has been an integral contribution to science. This innovative model more closely mimics the human menopause transition compared to the ovary-intact or Ovx models by effectively depleting the resting follicle pool but allowing for the retention of follicle-deplete ovarian tissue, resulting in an ovarian and hormone profile more similar to a vast majority of women undergoing the natural menopause transition who retain their reproductive organs in the post-reproductive life stage. In rodents, VCD is typically administered via a series of intraperitoneal injections ranging from 80 mg/kg-160 mg/kg [79–81, 94–96]. The Jackson Laboratory offers a VCD-treated mouse model available for purchase (https://www.jax.org/strain/100010), or researchers can administer the drug to rodents in the laboratory. Recently, an oral bait has been developed for rodents that contains a combination of VCD and triptolide, a Chinese herb thought to accelerate the follicular depletion of later-stage ovarian follicles (e.g., pre-ovulatory antral follicles) [83], which could be used both in the laboratory to decrease the number of necessary VCD injections and to target later-stage follicle development, as well as commercially to reduce wild rodent pest populations by further decreasing fertility. This model is still undergoing development but is an excellent addition to the VCD-related models [83]. The chief drawback of the VCD model is that it is toxic in high doses [82]. However, the use of VCD in the laboratory has been refined as a model of transitional menopause and no significant effects have been indicated on other organs under appropriate administration regimens [81, 94–95], although it may be an acute stressor to the animal during drug administration. Animals may decrease in body weight during injections, but generally return to baseline weights after drug administration.
Resulting serum hormone and gonadotropin profiles after VCD-induced follicular depletion have been shown to be similar to the hormone profiles observed in ovary-intact menopausal women [69, 79–80, 97–98]; it is noteworthy that gonadotropin and sex steroid hormone profiles from VCD-induced follicle depletion may vary based on species, dose, age, duration of administration, and length of time since VCD administration [95]. In addition to the circulating hormone profile produced by VCD, this is an excellent model of follicle atresia in women. Ovarian follicle counts are considered a reliable marker of reproductive capacity. In clinical research, antral follicle count (AFC) is often used as a biomarker for reproductive capacity in the context of fertility [99], and more recently, in the transition to menopause [100–102]. AFC may be a more accurate marker than circulating serum gonadal hormone and gonadotrophin levels, as these levels fluctuate erratically in the human menopause transition [101]. Because the VCD model allows for the retention of post-menopausal ovarian tissue, researchers can now evaluate how ovarian follicle count relates to reproductive senescence in rodents. This model can also inform our understanding of the impact of intact post-menopausal ovarian tissue with its continued release of androgens, particularly androstenedione (discussed in 4.1.3), [80] and the relationship to other low levels of circulating steroid hormones from extra-ovarian sources, such as the adrenals, breast, and adipose tissues in the post-menopausal life stage. Overall, the VCD menopause model has a substantial advantage over the ovary-intact and Ovx models in that it results in an ovarian follicle and circulating steroid hormone profile more similar to the majority of women transitioning into the post-menopausal life stage.
The VCD model is growing in popularity in the field of menopause and aging research. Our laboratory has implemented the VCD model in the context of spatial memory performance, and showed that transitional menopause differentially impacts spatial memory as well as the efficacy of the estrogenic HT, conjugated equine estrogens (CEE; tradename: Premarin) compared to Ovx animals [97–98]. Others have shown VCD-induced reproductive senescence is associated with insulin resistance, bone loss, atherosclerotic lesion development, and changes in anxiety-like behaviors [90, 103–105], all of which can be negative health risks related to aging and the menopause transition in women. These studies point to the benefits and utility of the VCD model, and suggest that it should be further tapped in the context of menopause research in order to elucidate novel therapies in an intact system.
4.1 Evaluating hormones, behavior, and brains in rodent menopause models
4.1.1 Standing on the shoulder of giants: building on classic frameworks of behavioral endocrinology research
Systematic investigations into hormones, brain, and behavior have a long history. Beginning in the early 20th century, gonadal hormones were found to be crucial not only for normal reproductive tract and mammary gland development, estrous cycles, and pregnancy, but also for the phenotypic display of sex-specific adult mating behaviors [106–110]. Soon after, Phoenix, Goy, Gerall, and Young presented the theory of organizational/activational effects of gonadal hormones on the body and brain in 1959, which suggested that critical periods exist for hormone exposures. Organizational effects were thought to be permanent and occur early in development to set up the brain to respond in a particular way later in life, while activational effects were considered to occur later in life and only when a particular hormone is present. In other words, later behaviors are activated based upon early organization of the brain and body systems [111–112]. Since these initial reports, we have learned that ovarian hormones have both organizational and activational effects on the female brain, and that related factors likely play into sensitivity and efficacy of HT during aging and reproductive senescence.
With this framework in mind, the field has continued to study hormone-brain-behavior interactions using rodent models in many dimensions and domains in the context of aging and menopause, including depression, anxiety, and cognition. We and many others have investigated the effects of dose, task-specific effects, type of hormone, menopause type, as well as route, timing, and duration of treatment administration on both brain and behavior in female rodents [43–45, 113–121].
4.1.2 Mazes and memory
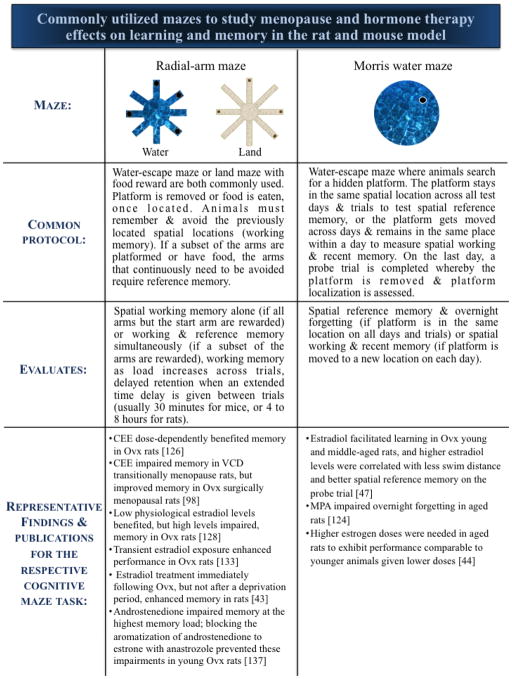
Mazes are a classic and effective way to study memory in the rodent and are sensitive to the effects of gonadal hormone changes associated with aging, Ovx, and follicular depletion. Multiple memory types can be assessed using maze paradigms, including reference memory, a form of long-term memory used to remember information that remains constant, and working memory, a type of short-term memory used to update information. Rodents have a general preference to navigate using spatial cues, so many maze tasks are geared toward allowing rodents to use their tendency to orient and remember in space by providing distal spatial cues around the room. For example, the standard Morris water maze (MM) tests spatial reference memory by requiring a rodent to learn to navigate to a hidden platform in a large tub of water by using spatial cues around the room. MM can also be used to evaluate overnight forgetting, a measure of memory retention, where performance on the last trial of the previous day is compared to performance on the first trial of the following day. The radial-arm maze and its water variant (water radial-arm maze, WRAM) assess spatial working and reference memory such that animals are tasked with finding several food rewards or hidden platforms that are not replaced within a day (Figure 6). This results in an increasing working memory load as trials progress; animals must remember where not to return after locating the reward within a day [113]. This measure of increasingly taxed working memory is especially key for translational menopause research. Often, verbal memory is impacted by the menopause transition and gonadal hormone loss, and in particular, the presence or absence of estrogens [122–123]. A challenge with animal models is that researchers cannot implement verbal memory tasks; however, a key part of these tests is remembering a particular number of items, so the memory load associated with the radial-arm maze paradigms is a similar measure that we can quantify using an animal model.
Figure 6.
Commonly utilized mazes to study menopause and hormone therapy effects on learning and memory in the rat and mouse model A comparison of common maze paradigms utilized with rodent models of menopause, and examples of representative results using the mazes.
4.1.3 Understanding hormone therapy effects on spatial memory and the brain using preclinical models
Our laboratory uses Ovx and VCD rat models to evaluate cognitive and brain changes associated with aging and the menopause transition, as well as how common estrogenic HT regimens, including 17β-estradiol, CEE, and progestogen components, such as progesterone and medroxyprogesterone acetate (MPA, a synthetic progestin that was combined with CEE in the WHI clinical trials, and is the main component in the birth control Depo Provera), impact spatial working and reference memory in water maze tasks. For example, we found that in middle-aged Ovx rats, MPA and progesterone impaired spatial working and reference memory on the WRAM, and that MPA exposure at any time point — whether early in adulthood, middle-age, or both stages — impaired memory, even when MPA was no longer detectable in circulation. This effect occurred specifically at the highest working memory load, where animals have the most items of information to remember; Ovx animals treated with MPA in middle-age also had impaired overnight forgetting on the MM, a measure of memory retention [124–125]. Related research utilizing the Ovx model showed that, depending on dose, CEE benefited spatial working and reference memory after a delay in the WRAM and a delayed match-to-sample task (DMS), another common water escape task used to evaluate spatial working and recent memory [126].
These behavioral effects were associated with observable brain changes. MPA decreased glutamic acid decarboxylase (GAD), the rate-limiting enzyme for the inhibitory neurotransmitter GABA, in the hippocampus and increased GAD in the entorhinal cortex, two regions important for learning and memory retention [124]. CEE increased the number of choline acetyltransferase (ChAT; the synthesizing enzyme for acetylcholine) – positive cells in the basal forebrain, and increased the neurotrophins brain derived neurotropic factor and nerve growth factor in the cingulate gyrus and perirhinal cortex [97, 126]. How then, can we reconcile some of the WHIMS findings? We showed that that beneficial effects of CEE are dependent upon menopause etiology. That is, Ovx animals administered CEE had enhanced spatial working and reference memory performance compared to control subjects on the WRAM and after a delay on the DMS, while VCD-treated animals given CEE had impaired performance compared to controls on the WRAM [98]. Importantly, more WRAM errors were positively correlated with higher serum androstenedione levels [98], an androgen and the primary hormone released by the post-menopausal ovary. Androstenedione can be aromatized to estrone, which is the primary estrogen component in CEE that was previously shown to impair memory at high doses [76]. These collective findings corroborated the WHIMS reports concerning the potential increased risk for memory impairment in the CEE+MPA groups, as women given that HT combination had a uterus, and likely experienced transitional menopause [34–35].
4.1.4 Optimizing parameters for hormone therapy: dose, timing, and healthy cognitive aging
Dose is key to elucidating estrogens’ effects; in Ovx rats, low physiological levels of estradiol benzoate (EB; similar to diestrus levels) benefitted performance on a land radial-arm maze, while higher doses of EB that resulted in high physiological levels (similar to proestrus levels) impaired performance [127–128]. Optimal dosing may also depend on age — Foster and colleagues reported that older animals required higher EB doses to achieve similar memory performance to younger animals receiving lower doses on a MM memory retention probe trial [44]. Importantly, rodent research supports the tenet that the aging brain can still respond to 17β-estradiol or EB treatment even after a period of deprivation, although sensitivity to hormone treatment may be reduced after a long withdrawal period [43, 129–132], pointing to a critical window of opportunity for HT early in the menopause transition. Although the presence of estrogen is thought to be necessary to benefit memory in middle age, the Daniel laboratory demonstrated that transient exposure to 17β-estradiol benefited spatial memory performance on the land radial-arm maze in middle-aged Ovx rats, even several months after hormone treatment ended [133]. Of note, estrogen-containing HT is not the only way to maintain or enhance cognitive performance during aging. For example, cognitive practice can mitigate age-related decline for a familiar task in males and females; in addition, females that have cognitive practice can transfer this enhanced performance to a novel task [134–135].
Rodent models of menopause have yielded invaluable insight into how aging and reproductive senescence impact cognition, allowing us to peer into critical windows of sensitivity to potentially maximize HT efficacy. Using these translational models, we can methodically test the effects of lifelong hormone exposures related to reproductive history, including endogenous hormone fluctuations (e.g., pregnancy, lactation) and exogenous hormone exposures (e.g., hormonal contraceptives, HT), to obtain a global view and better understanding of how these factors may interact and result in long-lasting effects on the trajectory of brain aging and reproductive senescence.
5.1 Conclusions
Since the early twentieth century, rodent models have been instrumental to our current understanding of the reproductive system, HPG feedback loops, and steroid hormone effects—particularly estrogens— on a wide range of systems, including cardiovascular disease, bone health, stroke, cancers, affective disorders, and cognitive processes, including spatial learning, memory, and attentional processes. In addition, we have the advantageous capacity to evaluate behavioral and brain changes at the cellular and molecular level much more quickly than would be possible using human subjects. This utility affords basic scientists the opportunity to explore novel brain targets and drug delivery methods [136] for favorable cognitive outcomes and healthy brain aging profiles that have the potential to translate to the clinical research setting.
These three core rodent models — ovary-intact, Ovx, and VCD — form the basis of our abilities as preclinical scientists to aid understanding of the female reproductive system, as well as brain and behavioral changes that occur with perturbations and alterations to this system, such as removal of steroid hormones, depletion of ovarian follicles, or exogenous HT treatment (Figure 7). Indeed, these models allow basic scientists to systematically and methodically evaluate longitudinal impacts of hormones and aging, as well as directly assess brain, ovarian, uterine, and other body tissues from which we can glean insight into the physiological correlates associated with the aging female and the menopause transition. All of these elements showcase the utility of rodent models as translational models of human menopause and aid in our understanding of risk factors associated with aging and menopause, providing direction and potential solutions for individualized treatments for women in mid-life to lower risk factors for disease and to maintain a high quality of life.
Figure 7.
Rodent Models of Menopause An illustration and comparison of three validated rodent models of menopause.
Highlights.
Frameworks and mechanisms of rodent and human reproductive senescence
Strengths and weaknesses of the most commonly used rodent menopause models
Testing cognition and the brain in rodent menopause models using a systems approach
Optimizing preclinical models for translation to promote healthy aging in women
Acknowledgments
Dr. Heather Bimonte-Nelson is funded by the following grant awards: NIA (AG028084), state of Arizona, and ADHS. No funding was received to write this article. We wish to express our sincere appreciation to Justin M. Palmer for his excellent assistance in formatting this document.
Abbreviations
- HT
hormone therapy
- VCD
4-vinylcyclohexene diepoxide
- WRAM
water radial-arm maze
- DMS
delayed match-to-sample task
Footnotes
Conflict of interest:
I, Heather A. Bimonte-Nelson, have no conflicts of interest to declare.
I, Stephanie V. Koebele, have no conflicts of interest to declare.
This was an invited review by Dr. Margaret Rees following Stephanie Koebele’s receipt of the New Investigator Award at the 2015 European Menopause and Andropause Congress in Madrid, Spain.
Contributions
I, Heather A. Bimonte-Nelson, declare that I participated in the writing of this review and that I have seen and approved the final version. I have no conflicts of interest to declare, and am funded by the following grant awards: NIA (AG028084), state of Arizona, and ADHS. I, Stephanie V. Koebele, declare that I participated in the writing of this review and that I have seen and approved the final version. I have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.North American Menopause Society. Menopause Practice A Clinician’s Guide. 5. 2014. [Google Scholar]
- 2.Hoffman BL, Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham FG, Calver LE. In: Williams Gynecology. 2. Fried A, Boyle PJ, editors. McGraw-Hill; New York: 2012. [Google Scholar]
- 3.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19(4):387–395. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Safi Z, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertil Steril. 2014;101:905–915. doi: 10.1016/j.fertnstert.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Hale G, Robertson D, Burger H. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol. 2014;142:121–131. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt P. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19:257–71. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neves-e-Castro M, Birkhauser M, Samsioe G, Lambrinoudaki I, Palacios S, Borrego R, et al. EMAS position statement: the ten point guide to the integral management of menopausal health. Maturitas. 2015;81:88–92. doi: 10.1016/j.maturitas.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 8.The World Factbook 2014–15 (Internet) Washington, DC: Central Intelligence Agency; 2015. (cited 2015 Aug 16) Available from: https://www.cia.gov/library/publications/the-world-factbook/ [Google Scholar]
- 9.Murray C, Barber R, Foreman K, Ozgoren A, Abd-Allah F, Abera S, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015 doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. US Census Bur. 2014 [Google Scholar]
- 11.Vincent GK, Velkoff VA. The next four decades the older population in the United States: 2010–2015. US Census Bur. 2010 [Google Scholar]
- 12.Bimonte-Nelson HA. Rodent mazes and memory: continuing the search for the engram. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Springer Publishing, Humana Press; New York: 2015. pp. 3–36. [Google Scholar]
- 13.Bellino F, Wise P. Nonhuman primate models of menopause workshop. Biol Reprod. 2003;68:10–18. doi: 10.1095/biolreprod.102.005215. [DOI] [PubMed] [Google Scholar]
- 14.Camus S, Ko W, Pioli E, Bezard E. Why bother using non-human primate models of cognitive disorders in translational research? Neurobiol Learn Mem. 2015;124:123–129. doi: 10.1016/j.nlm.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Roelfsema PR, Treue S. Basic neuroscience research with nonhuman primates: a small but indispensable component of biomedical research. Neuron. 2014;82:1200–1204. doi: 10.1016/j.neuron.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Van Esch E, Cline JM, Buse E, Wood CE, de Rijk EP, Weinbauer GF. Summary comparison of female reproductive system in human and the cynomolgus monkey (Macaca fascicularis) Toxicol Pathol. 2008;26:171S–172S. [Google Scholar]
- 17.NIA Aged Rodent Colonies Handbook (Internet) 2015 (cited 2015 Dec) Available from: https://www.nia.nih.gov/research/dab/aged-rodent-colonies-handbook.
- 18.Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol. 2010;71:132–43. doi: 10.1016/j.ando.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Wallace W, Kelsey T. Human ovarian reserve from conception to the menopause. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker TG. A quantitative and cytological study of germ cells in the human ovaries. Proc R Soc Lond Biol. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 21.Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15(6):707–724. doi: 10.1210/edrv-15-6-707. [DOI] [PubMed] [Google Scholar]
- 22.Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81:433–42. doi: 10.1530/jrf.0.0810433. [DOI] [PubMed] [Google Scholar]
- 23.Downs J, Wise P. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–8. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise PM. Neuroendocrine modulation and repercussions of female reproductive aging. J Phys Colloq. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- 25.Wise PM, Weiland NG, Scarbrough K, Sortino MA, Cohen IR, Larson GH. Changing hypothalamopituitary function: its role in aging of the female reproductive system. Horm Res. 1989;31:39–44. doi: 10.1159/000181084. [DOI] [PubMed] [Google Scholar]
- 26.Wise PMKM, Krajnak PM, Kashon ML. Menopause: the aging of multiple pacemarkers. Sci. 1996;273:67–70. doi: 10.1126/science.273.5271.67. [DOI] [PubMed] [Google Scholar]
- 27.Wise PM, Kashon ML, Krajnak KM, Rosewell KL, Cai A, Scarbrough KK, et al. Aging of the female reproductive system: a window into brain aging. Recent Prog Horm Res. 1997;52:279–305. [PubMed] [Google Scholar]
- 28.Wise P, Smith M, Dubal D, Wilson M, Krajnak K, Rosewell K. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20:243–248. doi: 10.1210/edrv.20.3.0364. [DOI] [PubMed] [Google Scholar]
- 29.Menopause: diagnosis and management. NICE guidelines [NG23] Published date: November 2015 https://www.nice.org.uk/guidance/ng23/resources/menopause-diagnosis-and-management-1837330217413.
- 30.Koebele SV, Bimonte-Nelson HA. Trajectories and phenotypes with estrogen exposures across the lifespan: What does Goldilocks have to do with it? Horm Beh. 2015;74:86–104. doi: 10.1016/j.yhbeh.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wise P, Suzuki S, Brown C. Estradiol: a hormone with diverse and contradictory neuroprotective actions. Dialogues Clin Neurosci. 2009;11:297–303. doi: 10.31887/DCNS.2009.11.3/pmwise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turgeon J, Carr M, Maki P, Mendelsohn M, Wise P. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- 33.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative memory study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 34.Shumaker S, Legault C, Rapp S, Thal L, Wallace R, Ockene J, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The women’s health initiative memory study: a randomized, controlled trial. Obstet Gynecol Surv. 2003;58:675. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 35.Shumaker S, Legault C, Kuller L, Rapp S, Thal L, Lane D, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: women’s health initiative memory study. Obstet Gynecol Surv. 2004;59:711. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 36.Coker L, Espeland M, Rapp S, Legault C, Resnick S, Hogan P, et al. Postmenopausal hormone therapy and cognitive outcomes: the women’s health initiative memory study (WHIMS) J Steroid Biochem Mol Biol. 2010;118:304–10. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm Behav. 2013;63(2):231–7. doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Maki PM. Hormone thearapy and cognitive function: is there a critical period for benefit? Neurosci. 2006;138:1027–103. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Maki P. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarrey AC, Resnick SM. Postmenopausal hormone therapy and cognition. Horm Behav. 2015;74:167–72. doi: 10.1016/j.yhbeh.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocca W, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7:163–166. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maki P, Dennerstein L, Clark M, Guthrie J, LaMontagne P, Fornelli D, et al. Perimenopausal use of hormone therapy is associated with enhanced memory and hippocampal function later in life. Brain Res. 2011;1379:232–243. doi: 10.1016/j.brainres.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniel J, Hulst J, Berbling J. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinol. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 44.Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs R. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 46.McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54(3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinol. 2012;153:3571–8. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Kempen T, Milner T, Waters E. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–187. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finch C. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol. 2014;142:132–41. doi: 10.1016/j.jsbmb.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 52.Burger HG. Physiology and endocrinology of the menopause. Medicine. 2006;34(1):27–30. [Google Scholar]
- 53.Clemens JA, Meites J. Neuroendocrine status of old constant-estrus rats. Neuroendocrinol. 1971;7:249–256. doi: 10.1159/000121973. [DOI] [PubMed] [Google Scholar]
- 54.Huang HH. Patterns of sex steroid and gonadotropin secretion in aging females rats. Endocrinol. 1978;103(5):1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- 55.Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 56.Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinol. 1990;126(2):884–90. doi: 10.1210/endo-126-2-884. [DOI] [PubMed] [Google Scholar]
- 57.Wilkes MM, Lu KH, Fulton SL, Yen SS. Hypothalamic-pituitary-ovarian interactions during reproductive senescence in the rat. Adv Exp Med Biol. 1978;113:127–47. doi: 10.1007/978-1-4684-8893-7_8. [DOI] [PubMed] [Google Scholar]
- 58.Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod. 1983;28(2):255–60. doi: 10.1095/biolreprod28.2.255. [DOI] [PubMed] [Google Scholar]
- 59.Johnson J, Canning J, Kaneko T, Pru J, Tilly J. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 60.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 61.Kermath BA, Gore A. Neuroendocrine control of the transition to reproductive senescence: lessons learned from the female rodent model. Neuroendocrinol. 2012;96:1–12. doi: 10.1159/000335994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wise PM. Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol, and progesterone concentrations in middle-aged rats. Life Sci. 1982a;31:165–173. doi: 10.1016/0024-3205(82)90429-5. [DOI] [PubMed] [Google Scholar]
- 63.Wise PM. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc Soc Exp Biol Med. 1982b;169(3):348–354. doi: 10.3181/00379727-169-41356. [DOI] [PubMed] [Google Scholar]
- 64.Cooper RL, Conn PM, Walker RF. Characterization of the LH surge in middle-aged female rats. Biol Reprod. 1980;23:611–615. doi: 10.1095/biolreprod23.3.611. [DOI] [PubMed] [Google Scholar]
- 65.Gore A, Oung T, Yung S, Flagg R, Woller M. Neuroendocrine mechanisms for reproductive senescence in the female rat. Endocr. 2000a;13:315–323. doi: 10.1385/ENDO:13:3:315. [DOI] [PubMed] [Google Scholar]
- 66.Gore AC, Yeung G, Morrison JH, Oung T. Neuroendocrine aging in the female rat: the changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-Methyl-D-Aspartate receptors. Endocrinol. 2000b;141(12):4757–4767. doi: 10.1210/endo.141.12.7841. [DOI] [PubMed] [Google Scholar]
- 67.Zuo Z, Mahesh VB, Zamorano PL, Brann DW. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: a possible role in reproductive aging? Endocrinology. 1996;137(6):2334–2338. doi: 10.1210/endo.137.6.8641183. [DOI] [PubMed] [Google Scholar]
- 68.Arias P, Carbone S, Szwarcfarb B, Feleder C, Rodríguez M, Scacchi P, Moguilevsky JA. Effects of aging on N-methyl-D-aspartate (NMDA)-induced GnRH and LH release in female rats. Brain Research. 1996;740:234–238. doi: 10.1016/s0006-8993(96)00862-1. [DOI] [PubMed] [Google Scholar]
- 69.Timiras P, Quay W, Vernadakis A. Horm Aging. Boca Raton: NY: CRC Press; 1995. [Google Scholar]
- 70.Barha CK, Galea LA. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. 2011;32(11):2091–2095. doi: 10.1016/j.neurobiolaging.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Gatewood J, Morgan M, Eaton M, McNamara I, Stevens L, Macbeth A, et al. Motherhood mitigates aging- related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Macbeth A, Scharfman H, MacLusky N, Gautreaux C, Luine V. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macbeth A, Luine V. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 74.Cost K, Lobell T, Williams-Yee Z, Henderson S, Dohanich G. The effects of pregnancy, lactation, and primiparity on object-in-place memory of female rats. Horm Behav. 2014;65:3239. doi: 10.1016/j.yhbeh.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Olson ME, Bruce J. Ovariectomy, ovariohysterectomy, and orchidemctomy in rodents and rabbits. Can Vet J. 1986;27(12):523–527. [PMC free article] [PubMed] [Google Scholar]
- 76.Engler-Chiurazzi EB, Talboom JS, Braden BB, Tsang CW, Mennenga S, Andrews M, et al. Continuous estrone treatment impairs spatial memory and does not impact number of basal forebrain cholinergic neurons in the surgically menopausal middle-aged rat. Horm Behav. 2012;62(1):1–9. doi: 10.1016/j.yhbeh.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. J Phys Colloq. 2004;229:977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- 78.Diz-Chaves Y, Kwiatkowska-Naqvi A, Von Hülst H, Pernía O, Carrero P, Garcia-Segura LM. Behavioral effects of estradiol therapy in ovariectomized rats depend on the age when the treatment is initiated. Exp Gerontol. 2012;47(1):93–9. doi: 10.1016/j.exger.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, et al. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 80.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71:130–138. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 81.Hoyer P, Devine P, Hu X, Thompson K, Sipes I. Ovarian toxicity of 4- vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol. 2001;29(1):91–99. doi: 10.1080/019262301301418892. [DOI] [PubMed] [Google Scholar]
- 82.National Toxicology Program (NTP) Toxicology and carcinogenesis studies of 4-vinylcyclohexene diepoxide. Tech Rep Ser. 1989;362 [PubMed] [Google Scholar]
- 83.Dyer C, Raymond-Whish S, Schmuki S, Fisher T, Pyzyna B, Bennett A, et al. Accelerated follicle depletion in vitro and in vivo in Sprague- Dawley rats using the combination of 4-vinylcyclohexene diepoxide and triptolide. J Zoo Wildl Med. 2013;44(4S):S9–S17. doi: 10.1638/1042-7260-44.4S.S9. [DOI] [PubMed] [Google Scholar]
- 84.Hu X, Christian P, Sipes I, Hoyer P. Expression and redistribution of cellular bad, bax, and Bcl-xL protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001;65(5):1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- 85.Hu X, Christian P, Thompson K, Sipes I, Hoyer P. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001;65(1):87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- 86.Borman SM, VanDePol BJ, Kao S, Thompson KE, Sipes IG, Hoyer PB. A single dose of the ovotoxicant 4-vinylcyclohexene diepoxide is protective in rat primary ovarian follicles. Toxicol Appl, Pharmacol. 1999;158:244–252. doi: 10.1006/taap.1999.8702. [DOI] [PubMed] [Google Scholar]
- 87.Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8(6):509–14. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 88.Hirshfield A. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 89.Kao S, Sipes I, Hoyer P. Early effects of ovotoxicity induced by 4- vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 1999;13(1):67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 90.Mayer L, Dyer C, Eastgard R, Hoyer P, Banka C. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler Throm Vasc Biol. 2005;25(9):1910–6. doi: 10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- 91.Springer L, Flaws J, Sipes I, Hoyer P. Follicular mechanisms associated with 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Reprod Toxicol. 1996;10(2):137–43. doi: 10.1016/0890-6238(95)02056-x. [DOI] [PubMed] [Google Scholar]
- 92.Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4- vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996b;139(2):394–401. doi: 10.1006/taap.1996.0180. 1996. [DOI] [PubMed] [Google Scholar]
- 93.Springer L, Tilly J, Sipes I, Hoyer P. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol. 1996;139(2):402–10. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- 94.Devine PJ, Sipes IG, Hoyer PB. Effect of 4-vinylcyclohexene diepoxide dosing in rats on GSH levels in liver and ovaries. Toxicol Sci. 2001;62(2):315–20. doi: 10.1093/toxsci/62.2.315. [DOI] [PubMed] [Google Scholar]
- 95.Frye JB, Lukefahr AL, Wright LE, Marion SL, Hoyer PB, Funk JL. Modeling perimenopause in Sprague Dawley rats by chemical manipulation of the transition to ovarian failure. Comp Med. 2012;62(3):193–202. [PMC free article] [PubMed] [Google Scholar]
- 96.Wright LE, Frye JB, Lukefahr AL, Marion SL, Hoyer PB, Besselsen DG, et al. 4-Vinylcyclohexene diepoxide (VCD) inhibits mammary epithelial differentiation and induces fibroadenoma formation in female Sprague Dawley rats. Reprod Toxicol. 2011;32(1):26–32. doi: 10.1016/j.reprotox.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, et al. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinol. 2009;150(9):4248–59. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinol. 2010;151(8):3795–804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.American College of Obstetricians and Gynecologists (ACOG), Ovarian reserve testing, Committee Opinion 618–2015.
- 100.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 101.Hansen K, Hodnett G, Knowlton N, Craig L. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 102.Hansen KR, Craig LB, Zavy MT, Klein NA, Soules MR. Ovarian primordial and non-growing follicle counts according to the stages of reproductive aging workshop (STRAW) staging system. Menopause. 2012;19(2):164–171. doi: 10.1097/gme.0b013e31823b0b2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reis F, Pestana-Oliveira N, Leite C, Lima F, Brandão M, Graeff F, et al. Hormonal changes and increased anxiety-like behavior in a perimenopause-animal model induced by 4-vinylcyclohexene diepoxide (VCD) in female rats. Psychoneuroendocrinology. 2014;49:130–140. doi: 10.1016/j.psyneuen.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 104.Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk ML, Bouxsein JL, et al. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res. 2008;23(8):1296–303. doi: 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero-Aleshire MJ, Diamond-Stanic MK, Hasty AH, Hoyer PB, Brooks HL. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2009;297:587–592. doi: 10.1152/ajpregu.90762.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parkes AS, Bellerby CW. Studies on the internal secretions of the ovary. J Physiol. 1926;61(4):562–75. doi: 10.1113/jphysiol.1926.sp002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parkes AS. The internal secretions of the ovary. Proc R Soc Med. 1927;20(10):1663–166. [PMC free article] [PubMed] [Google Scholar]
- 108.Beach FA. Female mating behavior shown by male rats after administration of testosterone propionate. Endocrinol. 1941;29:409–412. [Google Scholar]
- 109.Beach FA. Male and female mating behavior in pre-pubertally castrated female rats treated with androgens. Endocrinol. 1942;31:673–678. [Google Scholar]
- 110.Beach F. A review of physiological and psychological studies of sexual behavior in mammals. Physiol Reviews. 1947;27(2):240–307. doi: 10.1152/physrev.1947.27.2.240. [DOI] [PubMed] [Google Scholar]
- 111.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the guinea pig. Endocrinol. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 112.Young WC, Goy RW, Phoenix CH. Hormones and sexual behavior. Sci. 1964;143(3606):212–218. doi: 10.1126/science.143.3603.212. [DOI] [PubMed] [Google Scholar]
- 113.Bimonte-Nelson HA, Daniel JM, Koebele SV. The mazes. In: Bimonte-Nelson HA, editor. The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition. Springer Publishing, Humana Press; New York: 2015. pp. 37–72. [Google Scholar]
- 114.Chisholm NC, Juraska JM. Long-term replacement of estrogen in combination with medroxyprogesterone acetate improves acquisition of an alternation task in middle-aged female rats. Behav Neurosci. 2012;126(1):128–136. doi: 10.1037/a0026461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Daniel J, Hulst J, Lee C. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neurosci. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Frick K, Gresack J. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117(6):1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 117.Gibbs R. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- 118.Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnson DA, Zambon NJ, Gibbs RB. Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Res. 2002;943:132–41. doi: 10.1016/s0006-8993(02)02623-9. [DOI] [PubMed] [Google Scholar]
- 120.Nelson B, Witty C, Williamson E, Daniel J. A role for hippocampal actin rearrangement in object placement memory in female rats. Neurobiol Learn Mem. 2012;98:284–290. doi: 10.1016/j.nlm.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 121.Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progesterone infusions enhance object recognition in young female mice. Pharmacol Biochem Behav. 2009;93:177–182. doi: 10.1016/j.pbb.2009.05.012. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sherwin B. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 123.Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci. 2012;126:123–7. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, et al. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93:444–453. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braden BB, Garcia AN, Mennenga SE, Prokai L, Villa SR, Acosta JI, et al. Cognitive-impairing effects of medroxyprogesterone acetate in the rat: independent and interactive effects across time. Psychopharmacol. 2011;218:405–418. doi: 10.1007/s00213-011-2322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, et al. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2011;32(4):680–97. doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–8. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 128.Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116(5):928–34. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- 129.McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD, CD Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54(3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J Neurosci. 1999;19(9):3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;1800:1068–76. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Rodgers S, Bohacek J, Daniel J. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinol. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- 134.Markowska AL, Savonenko AV. Protective effect of practice on cognition during aging: implications for predictive characteristics of performance and efficacy of practice. Neurobiol Learn Mem. 2002;78(2):294–320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- 135.Talboom JS, West EB, Engler-Chiurazzi SG, Enders CK, Crain I, Bimonte-Nelson HA. Learning to remember: cognitive training-induced attenuation of age-related memory decline depends on sex and cognitive demand, and can transfer to untrained cognitive domains. Neurobiology of Aging. 2014;35:2791–2802. doi: 10.1016/j.neurobiolaging.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prokai L, Nguyen V, Szarka S, Garg P, Sabnis G, Bimonte-Nelson HA, et al. The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders. Sci Transl Med. 2015;7(297):297ra113. doi: 10.1126/scitranslmed.aab1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mennenga SE, Koebele SV, Mousa AA, Alderete TJ, Tsang CWS, Acosta JI, et al. Pharmacological blockade of the aromatase enzyme, but not the androgen receptor, reverses androstenedione-induced cognitive impairments in young surgically menopausal rats. Steroids. 2015;99:1625. doi: 10.1016/j.steroids.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]