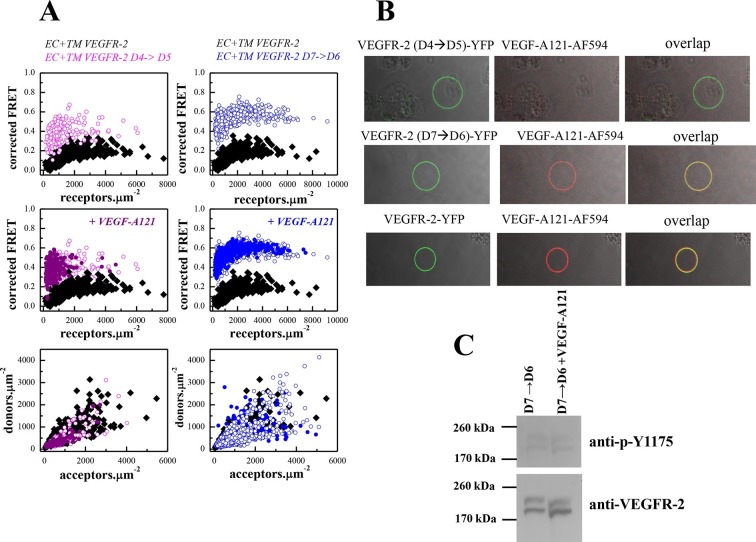
Figure 5. The D4 and D7 mutants do not respond to ligand.
(A) FRET measurements for the D4 and D7 mutants, in the absence of ligand and in the presence of VEGF-A121. The two mutants exhibit much higher FRET efficiencies, compared to the wild-type. The ligand, at 3 μg.ml-1, has no effect on receptor dimerization. (B) VEGF-A121 does not bind to the D4→D5 mutant, when it binds to the wild-type and the D7→D6 mutant. Here, a single vesicle containing YFP-tagged mutant receptors (two top rows) or wild-type receptors (bottom row) is shown after incubation with 3 μg.ml-1 AlexaFluor 594-labeled VEGF-A (VEGF-A121-AF594) (SibTech Inc., CT). Note the differences in the middle column, showing the fluorescence of the bound ligand. (C) Western blots comparing the phosphorylation of the full-length D7→D6 mutant in the absence and presence of VEGF-A121. The top band corresponds to the mature fully glycosylated form of VEGFR-2. The ligand does not increase the phosphorylation.
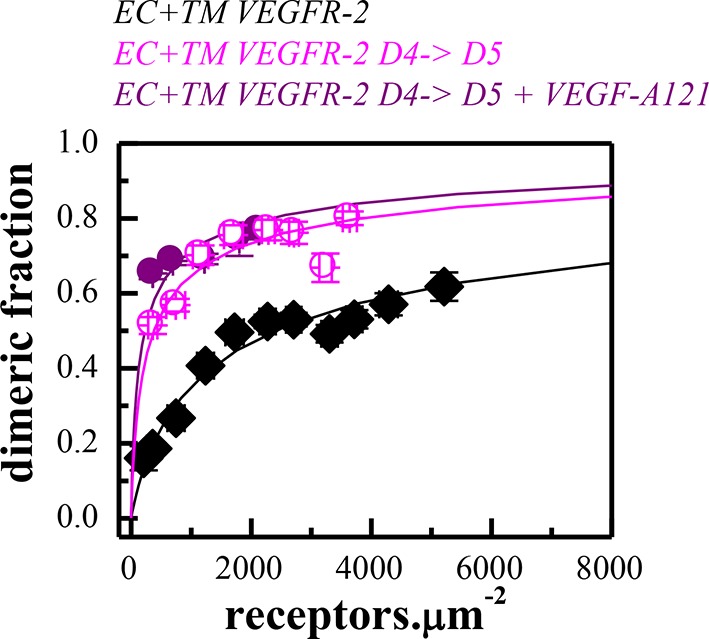
Figure 5—figure supplement 1. Dimerization curves for the wild-type VEGFR-2 in the absence of ligand, the D4→D5 mutant in the absence of ligand, and the D4→D5 mutant in the presence of VEGF-A121.