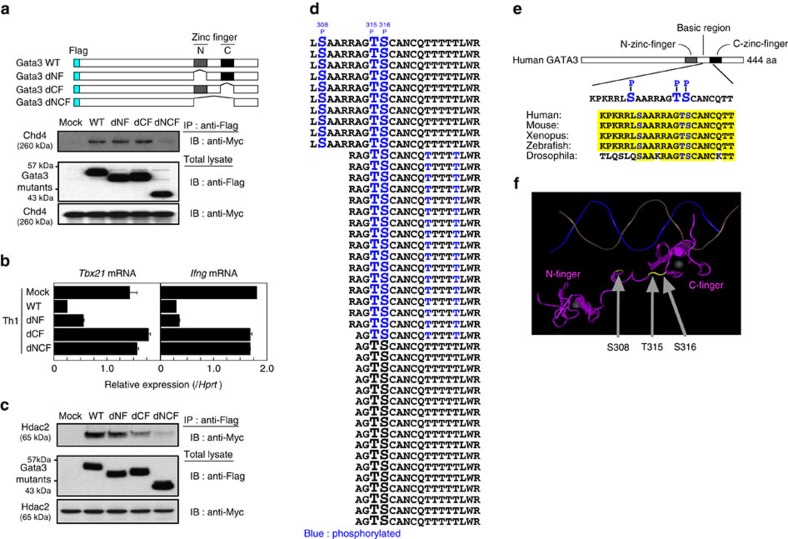
Figure 1. Identification of Gata3 phosphorylation in the C-terminal zinc finger.
(a) Schematic representations of the Flag-tagged Gata3 WT or deletion mutants are shown (top panel). Flag-tagged Gata3 WT, dNF, dCF or dNCF plasmid constructs were transfected with Myc-tagged Chd4 into 293T cells. Two days later, the amount of Myc-tagged Chd4 associated with the Flag-tagged WT or mutant Gata3 was assessed by immunoprecipitation (IP) followed by immunoblotting (IB) (middle panel). Total lysates were also subjected to IB in parallel (lower panel). (b) Naive CD4 T cells were stimulated under Th1 conditions and then infected with a retrovirus vector carrying WT or mutant Gata3 cDNA. Three days later, the retrovirus-infected GFP-expressing cells were purified and the levels of mRNA of Tbx21 and Ifng were measured by RT-qPCR. The relative expression (/Hprt) is shown with s.d.'s. (c) The amount of Myc-tagged Hdac2 associated with Flag-tagged Gata3 mutants were assessed as in Fig. 1a. (d) D10G4.1 cells were infected with a lentivirus encoding Flag-Gata3 and then the immunopurified Gata3 was subjected to a LC-MS/MS analysis to assess posttranslational modifications. All Gata3 peptides including Thr315 and Ser316 detected by our mass spectrometry analysis are shown. Blue characters indicate phosphorylated amino acids. (e) The phosphorylated residues of Gata3 in the linker region of tandem zinc fingers are highly conserved from Drosophila to human. (f) The 3D structure of Gata3 zinc fingers bound to DNA, including the novel phosphorylation sites (Ser308, Thr315 and Ser316) determined using the Molecular Modeling Database (MMDB ID; 105495)30, was drawn using the Cn3D software programme. The phosphorylated Ser/Thr residues are highlighted in yellow. Four (b) and three (a,c) independent experiments were performed with similar results.