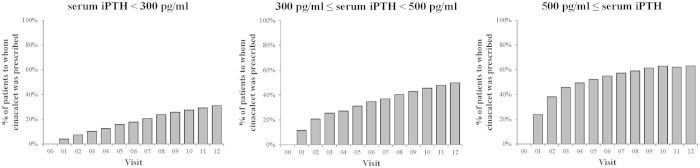
Figure 1. Changes in the proportion of patients receiving cinacalcet over the 3-year study period, stratified by baseline iPTH category.
. Changes in the proportion of patients receiving cinacalcet are shown for 3 categories of serum iPTH at baseline (n = 1,948 for <300 pg/ml, n = 824 for 300–<500 pg/ml, and n = 504 for ≥500 pg/ml). Visit 0 indicates the baseline (December 2007). The time between visits was 3 months. In January 2008 (within visit 1), cinacalcet was approved for use in clinical practice in Japan. Data were derived from the subcohort (n = 3,276). The numbers of patients analyzed gradually decreased to 2,469 at visit 12, due to death, loss to follow-up, and other reasons.