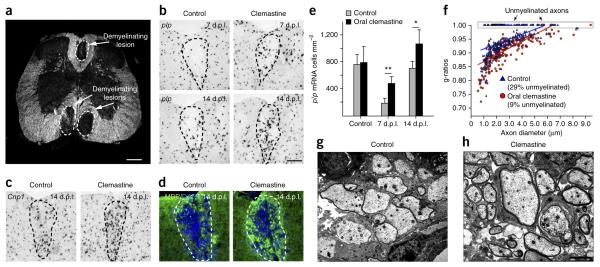
Figure 5.
Clemastine enhances the kinetics of remyelination and promotes remyelination in mice after gliotoxic injury with lysolecithin. (a) Dark-field micrograph of an adult mouse spinal cord illustrates focal demyelinated lesions in the dorsal funiculus and ventrolateral white matter. Scale bar, 300 μm. (b) Focal demyelinated lesions induced by injection of lysolecithin (n = 6) in mice treated with or without clemastine. In situ hybridization of plp in the lesions were examined after oral administration of clemastine at 7 and 14 days post lesion (d.p.l.). Scale bar, 100 μm. (c,d) Mice at 14 d.p.l. were subjected to Cnp1 in situ hybridization (c) and MBP staining (d) following the administration of clemastine and demyelination. Dashed lines demarcate lesion areas. Scale bar in b applies to c and d. (e) Quantification of plp in situ hybridization. Error bars represent mean ± s.d., and all experiments were performed in quadruplicate. *P = 0.05, **P = 0.009, significance based on Student’s t-test. (f) Quantification of myelin sheath thickness and the proportion of myelinated and unmyelinated axons in control (blue) and clemastine-treated (red) mice at d.p.l. by g-ratio analysis. The scatterplot displays g-ratios of individual axons as a function of axonal diameter. All g-ratios were analyzed from transmission electron microscopy images. (g,h) Representative electron microscopy images for control (g) and clemastine-treated (h) mice at 14 d.p.l. Scale bar, 2 μm.