Abstract
Background:
A limitation of bronchoscopic balloon dilatation (BBD) is that airflow must be completely blocked for as long as possible during the operation. However, the patient often cannot hold his or her breath for a long period affecting the efficacy of the procedure. In this study, we used an extra-small-diameter tube to provide assisted ventilation to patients undergoing BBD and assessed the efficacy and safety of this technique.
Methods:
Bronchoscopic balloon dilatation was performed in 26 patients with benign tracheal stenosis using an extra-small-diameter tube. The tracheal diameter, dyspnea index, blood gas analysis results, and complications were evaluated before and after BBD. Statistical analyses were performed by SPSS version 16.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results:
Sixty-three BBD procedures were performed in 26 patients. Dyspnea immediately improved in all patients after BBD. The tracheal diameter significantly increased from 5.5 ± 1.5 mm to 13.0 ± 1.3 mm (P < 0.001), and the dyspnea index significantly decreased from 3.4 ± 0.8 to 0.5 ± 0.6 (P < 0.001). There was no significant change in the partial pressure of oxygen during the operation (before, 102.5 ± 27.5 mmHg; during, 96.9 ± 30.4 mmHg; and after, 97.2 ± 21.5 mmHg; P = 0.364), but there was slight temporary retention of carbon dioxide during the operation (before, 43.5 ± 4.2 mmHg; during, 49.4 ± 6.8 mmHg; and after, 40.1 ± 3.9 mmHg; P < 0.001).
Conclusion:
Small-diameter tube-assisted BBD is an effective and safe method for the management of benign tracheal stenosis.
Keywords: Balloon Dilatation, Benign Tracheal Stenosis, Bronchoscopy, Tube
INTRODUCTION
During the past few decades, clinicians have faced difficulties in the treatment of benign central airway stenosis, particularly in dilatation of tracheal stenosis. Pulmonary physicians now have more choices for management of benign tracheal stenosis because of recent advances in interventional pulmonology, including neodymium-doped yttrium aluminum garnet (Nd: YAG) laser resection, argon plasma coagulation, electrocautery, stent implantation, cryotherapy, and bronchoscopic balloon dilatation (BBD).[1,2] BBD has been accepted as a safe and efficient strategy and is recommended as a first-line treatment in the management of benign tracheobronchial obstruction.[3,4] However, dilatation of tracheal stenosis with BBD requires complete blocking of the airflow for as long as possible, and weak patients must endure hypoxia for relatively long periods of time. Additionally, most patients cannot hold their breath for a long period affecting the efficacy of BBD.
We performed endotracheal tube dilatation for dilatation of benign tracheal stenosis in 12 patients under local anesthesia and bronchoscopic guidance from March 2010 to August 2011. This technique ensured sustainable ventilation for patients during dilatation procedures. Although we achieved the desired results, the procedure was associated with complications resulting in rhinal or buccal mucosal lesions as well as a very unsatisfactory subjective experience for the patients. Therefore, in the present study, we used an extra-small-diameter tube as a respiratory duct for tracheal dilatation with BBD under local anesthesia and assessed the efficacy and safety of this technique.
METHODS
Study population
We reviewed the clinical information of 26 patients with benign tracheal obstruction who underwent BBD using an extra-small-diameter tube at Department of Respiratory Medicine, First Affiliated Hospital of Guangxi Medical University from June 2011 to November 2014. The patients included 16 men and 10 women with a mean age of 37.0 ± 17.3 years (range, 16–84 years). The tracheal obstructions were caused by endotracheal intubation or tracheotomy in 24 patients (92.3%) and by tracheal tuberculosis in 2 patients (7.7%). The stenosis sites included the upper trachea in 18 patients, the upper and middle trachea in 3, the middle trachea in 4, and the entire trachea plus the right main bronchus in 1. Eight of the 26 patients had mild to severe tracheomalacia.
Ethics approval
This study was approved by the Ethical Review Committee of First Affiliated Hospital of Guangxi Medical University (Approval No. 2012 [KY-E-004]).
Instruments
We chose a flexible fiberoptic bronchoscope (model BF-260; Olympus, Tokyo, Japan) as the therapeutic endoscope and prepared another flexible bronchoscope (model BF-1T60; Olympus) for emergencies. The guide wire used in the procedures had a diameter of 0.85 mm and length of 180 mm (Qiuhong Medical Equipment Co., Changzhou, China), and the balloon catheter had a diameter of 14–16 mm and a length of 110 mm (Qiuhong Medical Equipment Co.). The inflation device was manufactured by Shenzhen Ant Hi-Tech Industrial Co., Ltd., (Shenzhen, China). The respiratory tube used for assisted ventilation was made of polycarbonate material or cut from a tracheal stent delivery system (Micro-Tech [Nanjing] Co., Ltd., Nanjing, China) and had a length of 30 cm and lumen diameter of 2.5–3.5 mm. A trial test in which a disposable syringe was used to simulate the trachea showed no obvious deformation of the tube at a pressure of 700 kPa.
Preoperative preparation
Preoperative preparation involved determination of each patient's coagulation function, platelet count, blood gas analysis results, electrocardiographic findings, blood pressure, and dyspnea index using the American Thoracic Society dyspnea rating criteria.[5] High-resolution computed tomography of the neck and chest, tracheobronchial three-dimensional reconstruction, and diagnostic bronchoscopy were performed to determine the extent of the tracheobronchial obstruction and for selection of the optimal tube and balloon size. All patients provided informed consent and underwent topical anesthesia (2% lidocaine) with an aerosol spray before the procedures. All procedures were performed under the following conditions: Oxygen flow of 3–5 L/min, electrocardiographic monitoring, and pulse oxyhemoglobin saturation monitoring. When clinically possible, blood gas analysis was performed 1 h before the procedure, during the procedure, and 2 h after the procedure.
Bronchoscopic balloon dilatation with extra-small-diameter tube
Diagnostic bronchoscopy was repeated to localize the airway obstruction and assess the proximal and distal extent of the obstruction. A guide wire was then inserted through the working channel of the flexible bronchoscope and passed through the tracheal stenosis. For distal obstructions of 3–4 mm, the guide wire was held in place over the obstruction while the bronchoscope was withdrawn and reinserted next to the guide wire so that the obstruction could be adequately visualized. For distal obstructions of 2–3 mm, a tube was passed over and along the guide wire and held in place over the obstruction. Importantly, insertion of the extra tube into the main bronchus was avoided to prevent unilateral pulmonary ventilation. The guide wire was then withdrawn, reinserted through the working channel of the flexible bronchoscope, and passed through the trachea. An appropriately sized balloon catheter was then advanced over the guide wire and positioned within the obstruction. Once the correct position was confirmed, the balloon was inflated using a balloon inflation device with 100–500 kPa of pressure and held at that pressure for 30–180 s. If the oxyhemoglobin saturation was ≤90% or the patient could not endure hypoxia, the BBD procedure was immediately stopped, and the balloon was deflated and removed. The initial results were immediately observed after the procedure, and the postoperative airway diameter of the obstruction was evaluated. Inflation was usually performed three to five times to obtain the desired results. Electrocautery or argon plasma coagulation was required in certain patients prior to BBD. Aerosol inhalation of budesonide (Budesonide Inhalation Suspension; AstraZeneca, London, UK) was recommended to prevent restenosis after each procedure.
Statistical analysis
The tracheal diameter, dyspnea index, and blood gas analysis results (PaO2 and PaCO2) are expressed as mean ± standard deviation (SD). Categorical variables were analyzed using the Chi-square test, and paired-samples of continuous variables were analyzed using the paired-sample t-test. Comparison of multiple means was performed by univariate analysis using repeated-measures analysis of variance (ANOVA). All reported P values are two-sided, and statistical significance was defined as P ≤ 0.05. All analyses were performed using SPSS version 16.0 for Windows (SPSS, Inc., Chicago, IL, USA).
RESULTS
Demographic and etiologic characteristics
Twenty-six patients aged 16–84 years were included in our study. All 26 patients were diagnosed with tracheobronchial obstruction. Twenty-four patients (92.3%) had iatrogenic tracheal stenosis due to prolonged tracheal intubation or tracheotomy, and the remaining 2 (7.7%) had tracheobronchial tuberculosis. Symptomatic manifestations such as dyspnea, stridor, or coughing were present in all patients and were the main reasons for hospitalization. The locations of the tracheobronchial obstructions were as follows: Upper trachea in 18 patients, mid-trachea in 3, upper and mid-trachea in 4, and entire trachea in 1.
Initial bronchoscopic balloon dilatation outcomes
The immediate post-BBD changes in the tracheal diameter, dyspnea index, and blood gas analysis results are shown in Table 1. Among all procedures, the tracheal diameter significantly increased from 5.5 ± 1.5 mm to 13.0 ± 1.3 mm (P < 0.001), and the dyspnea index significantly decreased from 3.4 ± 0.8 to 0.5 ± 0.6 (P < 0.001). More importantly, the blood gas analysis results showed no dramatic change in PaO2 (before, 102.5 ± 27.5 mmHg; during, 96.9 ± 30.4 mmHg; and after, 97.2 ± 21.5 mmHg; P = 0.364). A side effect during the procedure was slight temporary retention of carbon dioxide (before, 43.5 ± 4.2 mmHg; during, 49.4 ± 6.8 mmHg; and after, 40.1 ± 3.9 mmHg; P < 0.001) [Table 1]. Typical BBD images are shown in Figures 1–4].
Table 1.
Changes in lumen diameter, dyspnea index, PaO2, and PaCO2
| Groups | Lumen diameter (mm) | Dyspnea index | PaO2 (mmHg) | PaCO2 (mmHg) |
|---|---|---|---|---|
| Before | 5.5 ± 1.5 | 3.4 ± 0.8 | 102.5 ± 27.5* | 43.5 ± 4.2† |
| During | − | − | 96.9 ± 30.4* | 49.4 ± 6.8† |
| After | 13.0 ± 1.3 | 0.5 ± 0.6 | 97.2 ± 21.5* | 40.1 ± 3.9† |
| t/F | −33.406 | 28.867 | 1.018 | −52.428 |
| P | 0.000 | 0.000 | 0.364 | 0.000 |
*Pairwise comparisons of before, during, and after; all P > 0.05; †Pairwise comparisons of before, during, and after; all P < 0.05. PaO2: Partial pressure of oxygen; PaCO2: Partial pressure of carbon dioxide.
Figure 1.
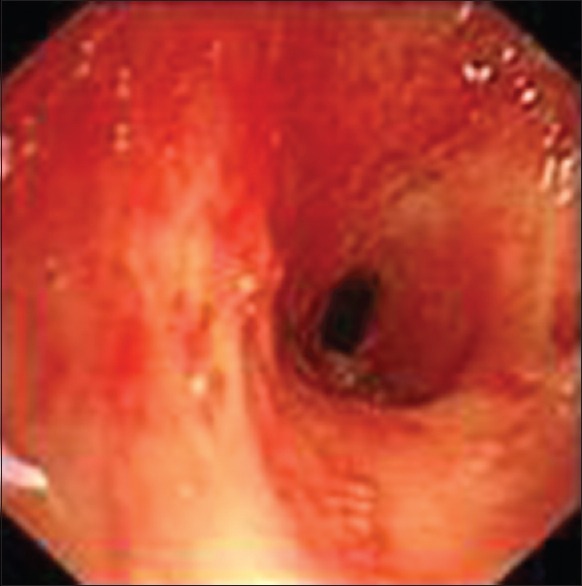
Severe tracheal stricture under bronchoscopic visualization.
Figure 4.
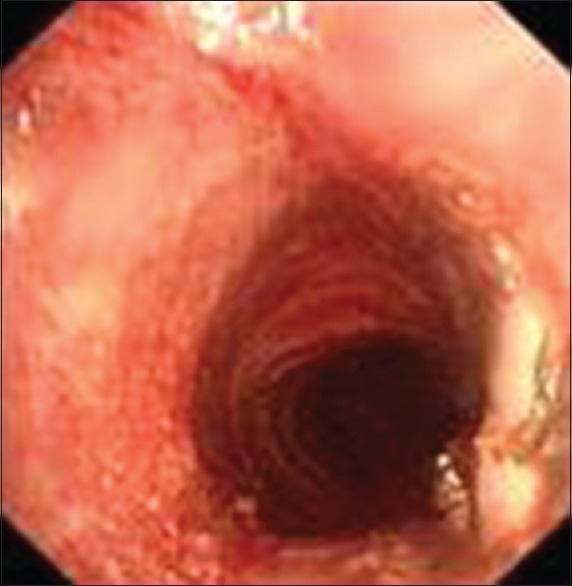
Trachea with a larger diameter after bronchoscopic balloon dilatation.
Figure 2.
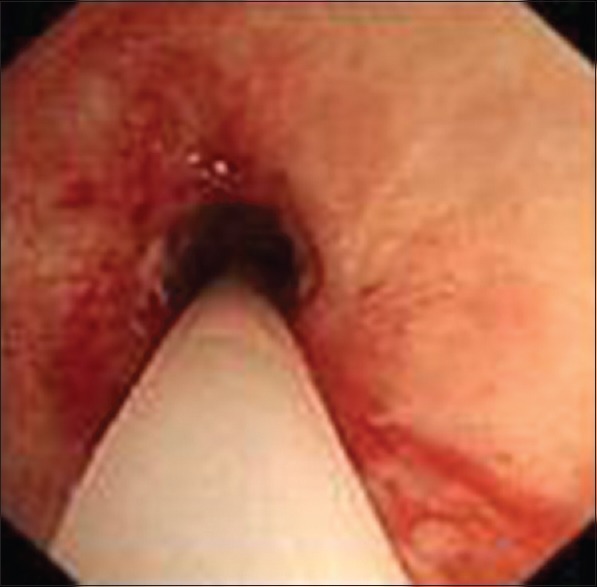
Assisted ventilation tube was retained within the trachea.
Figure 3.
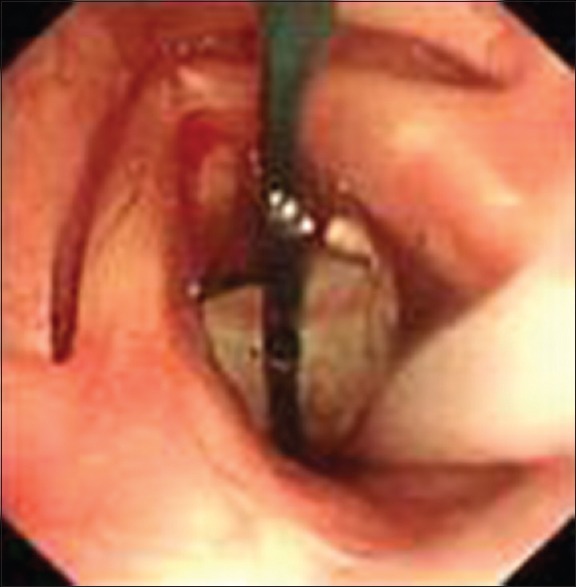
Assisted ventilation tube and balloon catheter were retained within the trachea.
Complications and related treatments of 63 procedures
The complications related to the procedures are as follows. In 44 procedures, the patients experienced mild chest pain after treatment but recovered 1–2 days later without interventional therapy. In 46 procedures, the patients developed mild tracheal bleeding, which was treated with intratracheal instillation of epinephrine at a concentration of 1:10,000. Superficial tracheal laceration of the anterior or posterior tracheal wall occurred in 23 patients, resulting in mild pneumomediastinum. All 23 patients were symptomatic and recovered 1 week later without specific treatment. Respiratory tract infection occurred in 17 patients after the procedures and required antibiotic treatment. Convulsions occurred in two patients during the procedure and lasted 5–10 s, but all recovered upon immediate cessation of the procedure. Two patients developed arrhythmia, which was resolved immediately after the procedure. Only the tracheal laceration was directly related to the BBD.
DISCUSSION
With the development of intensive care medicine and an increasing number of patients requiring admission to the intensive care unit in China, endotracheal intubation and tracheotomy have become widely performed in the medical emergency settings. Thus, the complications associated with endotracheal intubation and tracheotomy such as benign tracheal stenosis, have been markedly increasing year to year. In many previous reports on benign tracheal stenosis, endotracheal intubation and tracheotomy are the most common causes of benign tracheal stenosis.[2,6] The estimated incidence of severe postintubation and posttracheotomy tracheal stenosis in the general population is 4.9 cases per million per year.[7,8] In the present study, 24 (92.3%) tracheal stenoses developed after intubation or tracheotomy, and the remaining 2 (7.7%) were caused by tracheobronchial tuberculosis.
Balloon dilatation for the treatment of tracheobronchial stenosis was first used and reported by Cohen et al. in 1984.[9] BBD is an effective, safe, and easily performed interventional strategy for managing benign bronchial stenosis. BBD can also be combined with several other techniques to treat benign bronchial stenosis,[10] such as Nd: YAG laser treatment, cryotherapy, electrocautery, and stent placement. However, when performing BBD for tracheal stenosis dilatation, the trachea must be completely blocked, and weak patients are required to endure hypoxemia and carbon dioxide retention. This is an unavoidable clinical problem, especially in patients with severe tracheal stenosis; therefore, there are few reports on tracheal stenosis dilatation by BBD. In a previous study and in our clinical practice, most patients with tracheal stenosis endured hypoxemia for no more than 30–60 s, which is much shorter than the time required to reach the desired result. Questions remain on how to simultaneously solve this problem and ensure the safety and effectiveness of the procedure.
In our clinical practice, we found that patients with tracheal stenosis resulting in a lumen diameter of 3–6 mm can maintain an oxygen saturation of >90% at an oxygen inhalation rate of 3 L/min while resting. Based on this clinical observation, we introduced a small-diameter tube into the balloon dilatation procedure as a channel for assisted ventilation. Sixty-three procedures involving 306 effective balloon inflations were performed in 26 patients. Effective balloon inflation was defined as inflation of the balloon to the desired pressure for ≥30 s. Remarkable improvements in the tracheal diameter, dyspnea index, and ventilation were achieved immediately after the procedures. The initial cure rate was 100%. Some advantages of our new dilatation strategy are obvious. First, in our dilatation procedures, the extra tube acts as a temporary ventilation channel and effectively ensures the patient's oxygen supply because complete blockage of the airway is not necessary. Thus, the patients need not endure long periods of hypoxia, and the prolonged inflation time is safer and increases the chance of obtaining the desired result. Continuous airflow was observed in the tubes during our procedures. The blood gas analysis results showed no dramatic change in PaO2 (P = 0.364). Most patients had no clinical manifestations of hypoxia and maintained an oxyhemoglobin saturation of >90%. These results are completely different from those of a previous report in which the PaO2 decreased by 8–20 mmHg during interventional procedures.[11] Thus, we were able to extend the duration of each inflation for a relatively long time (range, 30–180 s; mean, 87.9 ± 40.8 s) while using a lower balloon pressure of 100–500 kPa. This is another advantage of our procedure in contrast to published reports by Lee et al.[6] and Low et al.,[12] in which BBD procedures were performed with a shorter inflation time (10–20 s) and higher balloon pressure (500–800 kPa). We have obtained similar ventilation effects via rigid bronchoscopy and a laryngeal mask airway.[13,14,15] This technique may represent a novel approach to the management of benign tracheal stenosis under local anesthesia.
Two studies performed by Kim et al.[16,17] reported that tracheobronchial laceration was a common complication of balloon dilatation, occurring in 51.6% of cases (64/124; 60 were superficial and 4 were deep). During follow-up, the median cumulative airway patency period in patients with and without tracheobronchial laceration was 24 and 4 months, respectively. Thus, it seems that luminal rupture promoted long-term curative effects. However, deep laceration increases the risk of life-threatening pneumomediastinum and bleeding. In our study, 23 procedures were associated with superficial tracheal laceration, but no deep laceration occurred. The absence of the deep laceration in our procedures may be explained by the low balloon pressure we adopted.
Although our procedure has shown initial success, there are two major limitations to our technique. Using our new inflation strategy, we observed that carbon dioxide retention occurred to some extent during most procedures. On the other hand, despite the 100% initial cure rate, we cannot completely eliminate the problem of restenosis. The major cause of restenosis is granulation tissue formation and subsequent fibrosis.[18] Compared with aerosol inhalation of budesonide in our study, some studies have reported the utility of mitomycin-C, high-dose-rate endobronchial brachytherapy, and glucocorticoid injection to prevent restenosis.[18,19,20] Additional studies involving a series of tubes with different diameters, models of ventilation, and application of multiple endoscopic therapies should be performed to determine the most appropriate tube size and length with respect to minimizing carbon dioxide retention and reducing the restenosis rate.
In conclusion, flexible BBD with a small-diameter tube is an effective, safe, novel strategy for the management of benign tracheal stenosis. However, close attention should be given to the risk of adverse effects caused by carbon dioxide retention, and application of multiple endoscopic therapies is essential for good long-term treatment effects.
Footnotes
Edited by: Li-Min Chen
Source of Support: This study was supported by a grant from Scientific Research and Technological Development Fund of Guangxi, China (No. 12300015).
Conflict of Interest: None declared.
REFERENCES
- 1.Hsia D, Musani AI. Interventional pulmonology. Med Clin North Am. 2011;95:1095–114. doi: 10.1016/j.mcna.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Galluccio G, Lucantoni G, Battistoni P, Paone G, Batzella S, Lucifora V, et al. Interventional endoscopy in the management of benign tracheal stenoses: Definitive treatment at long-term follow-up. Eur J Cardiothorac Surg. 2009;35:429–33. doi: 10.1016/j.ejcts.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 3.Mayse ML, Greenheck J, Friedman M, Kovitz KL. Successful bronchoscopic balloon dilation of nonmalignant tracheobronchial obstruction without fluoroscopy. Chest. 2004;126:634–7. doi: 10.1378/chest.126.2.634. [DOI] [PubMed] [Google Scholar]
- 4.Díaz-Jimenez J, Lisbona RL. Benign tracheal and bronchial stenosis. In: Díaz-Jimenez J, Rodriguez AN, editors. Interventions in Pulmonary Medicine. New York: Springer; 2013. pp. 165–84. [Google Scholar]
- 5.Stulbarg MS, Admas L. Philadelphia: Saunders; 1994. Textbook of Respiratory Medicine; pp. 511–2. [Google Scholar]
- 6.Lee WH, Kim JH, Park JH. Fluoroscopically guided balloon dilation for postintubation tracheal stenosis. Cardiovasc Intervent Radiol. 2013;36:1350–4. doi: 10.1007/s00270-013-0556-8. [DOI] [PubMed] [Google Scholar]
- 7.Zias N, Chroneou A, Tabba MK, Gonzalez AV, Gray AW, Lamb CR, et al. Post tracheostomy and post intubation tracheal stenosis: Report of 31 cases and review of the literature. BMC Pulm Med. 2008;8:18. doi: 10.1186/1471-2466-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouraei SA, Ma E, Patel A, Howard DJ, Sandhu GS. Estimating the population incidence of adult post-intubation laryngotracheal stenosis. Clin Otolaryngol. 2007;32:411–2. doi: 10.1111/j.1749-4486.2007.01484.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MD, Weber TR, Rao CC. Balloon dilatation of tracheal and bronchial stenosis. AJR Am J Roentgenol. 1984;142:477–8. doi: 10.2214/ajr.142.3.477. [DOI] [PubMed] [Google Scholar]
- 10.Shitrit D, Kuchuk M, Zismanov V, Rahman NA, Amital A, Kramer MR. Bronchoscopic balloon dilatation of tracheobronchial stenosis: Long-term follow-up. Eur J Cardiothorac Surg. 2010;38:198–202. doi: 10.1016/j.ejcts.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 11.Jolliet P, Chevrolet JC. Bronchoscopy in the intensive care unit. Intensive Care Med. 1992;18:160–9. doi: 10.1007/BF01709240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low SY, Hsu A, Eng P. Interventional bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir J. 2004;24:345–7. doi: 10.1183/09031936.04.00003604. [DOI] [PubMed] [Google Scholar]
- 13.Dincq AS, Gourdin M, Collard E, Ocak S, D’Odémont JP, Dahlqvist C, et al. Anesthesia for adult rigid bronchoscopy. Acta Anaesthesiol Belg. 2014;65:95–103. [PubMed] [Google Scholar]
- 14.Vorasubin N, Vira D, Jamal N, Chhetri DK. Airway management and endoscopic treatment of subglottic and tracheal stenosis: The laryngeal mask airway technique. Ann Otol Rhinol Laryngol. 2014;123:293–8. doi: 10.1177/0003489414525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrella F, Borri A, Casiraghi M, Cavaliere S, Donghi S, Galetta D, et al. Operative rigid bronchoscopy: Indications, basic techniques and results. Multimed Man Cardiothorac Surg 2014. 2014:1–6. doi: 10.1093/mmcts/mmu006. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Shin JH, Shim TS, OH YM, Song HY. Deep tracheal laceration after balloon dilation for benign tracheobronchial stenosis: Case reports of two patients. Br J Radiol. 2006;79:529–35. doi: 10.1259/bjr/17839516. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Shin JH, Song HY, Shim TS, Ko GY, Yoon HK, et al. Tracheobronchial laceration after balloon dilation for benign strictures: incidence and clinical significance. Chest. 2007;131:1114–7. doi: 10.1378/chest.06-2301. [DOI] [PubMed] [Google Scholar]
- 18.Tendulkar RD, Fleming PA, Reddy CA, Gildea TR, Machuzak M, Mehta AC. High-dose-rate endobronchial brachytherapy for recurrent airway obstruction from hyperplastic granulation tissue. Int J Radiat Oncol Biol Phys. 2008;70:701–6. doi: 10.1016/j.ijrobp.2007.07.2324. [DOI] [PubMed] [Google Scholar]
- 19.Simpson CB, James JC. The efficacy of mitomycin-C in the treatment of laryngotracheal stenosis. Laryngoscope. 2006;116:1923–5. doi: 10.1097/01.mlg.0000235934.27964.88. [DOI] [PubMed] [Google Scholar]
- 20.Oh SK, Park KN, Lee SW. Long-term results of endoscopic dilatation for tracheal and subglottic stenosis. Clin Exp Otorhinolaryngol. 2014;7:324–8. doi: 10.3342/ceo.2014.7.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]


