Introduction
Cardiac positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose (FDG) is often used for the diagnosis of sarcoidosis and an increase in FDG uptake in isolated segments or a patchy distribution is considered to represent inflammation.1, 2 However, heterogeneous uptake of FDG may be associated with other inflammatory myocardial diseases. We describe a case of infectious myocarditis with imaging findings identical to cardiac sarcoidosis.
Case Summary
A 34 year old Indian male with dyspnea, lung lesions and reduced left ventricular ejection fraction (LVEF) of 33% by echocardiography was referred for PET imaging to rule out sarcoidosis. Resting myocardial perfusion PET imaging with Rb82 followed by FDG-PET showed perfusion defects involving the basal to mid anteroseptum and inferoseptum which were FDG avid (Figure 1). Diffuse FDG avid lesions were also seen in the lung, spleen and cervical and mediastinal lymph nodes on whole body FDG PET-CT (Figure 2). Wall thinning, hypokinesis and scar involving the mid anteroseptum and inferoseptum were seen by cardiac magnetic resonance imaging (CMR) (Figure 3). Endobronchial biopsy revealed necrotizing granulomas, a pathologic finding suggestive of tuberculosis. A review of his past medical history revealed that he had been treated with adalimumab for psoriasis for the past 2 years. He had been prescribed a 9-month course of isoniazid before treatment with adalimumab for a positive PPD; however, he had been non-compliant with isoniazid therapy. He also reported having a positive rapid influenza test a month prior to his hospitalization. Based on the history and histopathologic findings consistent with tuberculosis, he was treated with anti-fungals and standard heart failure therapy for tuberculous myocarditis. Follow up echocardiography showed an LVEF of 56% with resolution of wall motion abnormalities.
Figure 1.
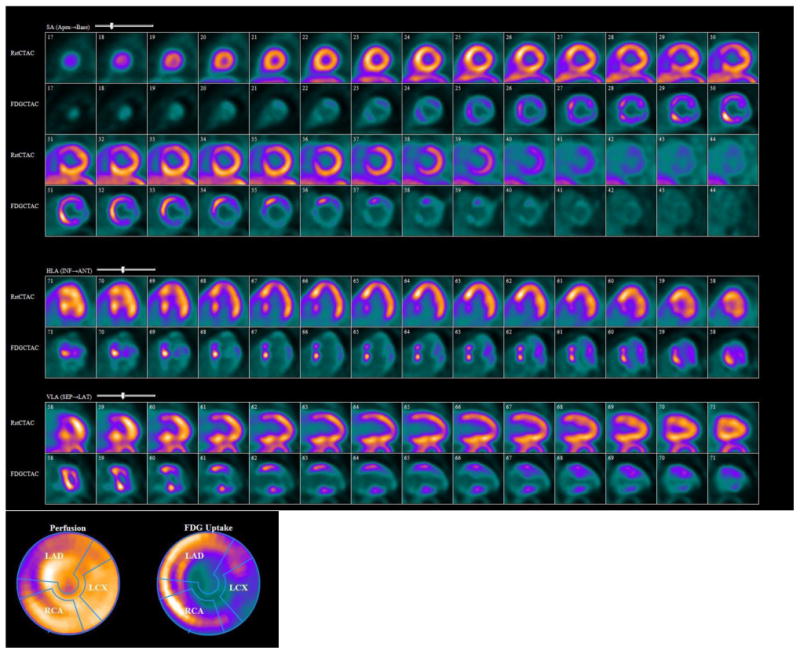
FDG-PET showing a resting perfusion defect in thebasal and mid anteroseptum and iferoseptum with a corresponding increase in FDG uptake.
Figure 2.

Whole body PDG PET-CT showing (A) FDG avid, diffuse nodular and ground glass opacities in the right upper lobe, patchy nodular opacities in the bilateral lower lobes of the lungs and mediastinal lymph nodes. (B) Lower lung and splenic lesions were also FDG avid.
Figure 3.
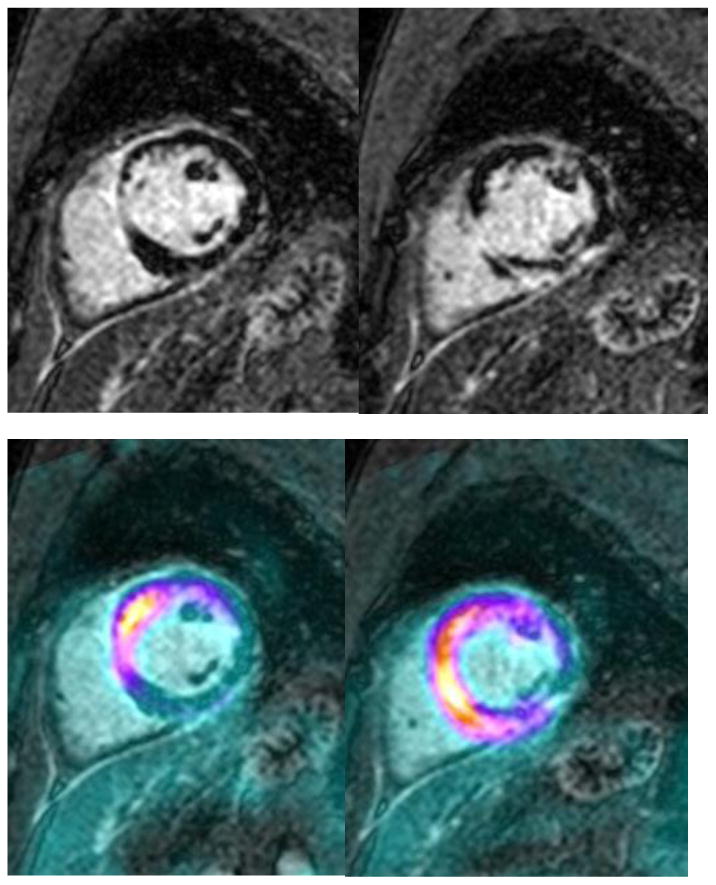
(A) CMR showing basal septal thinning and delayed gadolinium enhancement consistent with fibrosis. (B) Retrospective fusion of FDG-PET with delayed-enhancement MRI images. Note the location of greatest intensity (reflecting inflammation) in the anteroseptum and inferoseptum, in similar areas to those of delayed enhancement.
Conclusion
Tuberculous 3 and viral myocarditis4 should be included in the differential in patients with heterogeneous FDG uptake on PET imaging performed for diagnosis of infiltrative cardiomyopathy. Unlike tuberculosis and sarcoidosis, viral myocarditis does not typically manifest with perfusion defects. Given the similar appearance of myocardial FDG uptake in sarcoidosis and tuberculosis by PET imaging, a detailed medical history and histologic correlation are essential for differentiating tuberculous myocarditis and sarcoidosis.
Footnotes
Conflict of Interest: all authors do not report any conflicts of interest
Contributor Information
Brett W. Sperry, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH.
Jorge D. Oldan, Imaging Institute, Department of Radiology, Cleveland Clinic, Cleveland, OH.
Eileen M. Hsich, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH.
Balaji K. Tamarappoo, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH.
References
- 1.Tahara N, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, Baba K, Ishibashi M, Hayabuchi N, Narula J, Imaizumi T. Heterogeneous myocardial fdg uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–1228. doi: 10.1016/j.jcmg.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syed FF, Ntsekhe M, Gumedze F, Badri M, Mayosi BM. Myopericarditis in tuberculous pericardial effusion: Prevalence, predictors and outcome. Heart. 2014;100:135–139. doi: 10.1136/heartjnl-2013-304786. [DOI] [PubMed] [Google Scholar]
- 4.Sarda L, Colin P, Boccara F, Daou D, Lebtahi R, Faraggi M, Nguyen C, Cohen A, Slama MS, Steg PG, Le Guludec D. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol. 2001;37:786–792. doi: 10.1016/s0735-1097(00)01201-8. [DOI] [PubMed] [Google Scholar]


