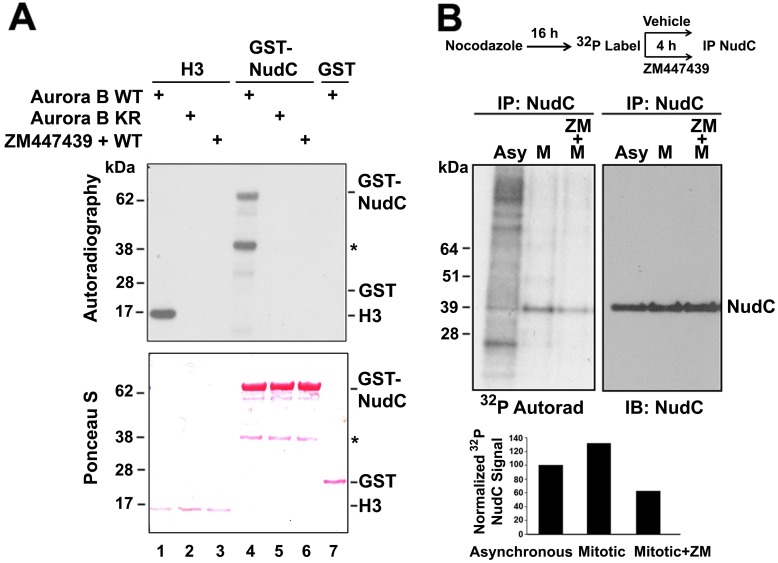
Fig 3. NudC is phosphorylated by Aurora B in vitro and in vivo.
(A) HeLa cells were transfected with FLAG-Aurora B wild type (WT) or a kinase dead (K106R) mutant Aurora B for 24 h. Aurora B was immunoprecipitated using anti-FLAG antibody and used in IP kinase assays. Substrates used were GST-NudC (lanes 4–6), histone H3 (lanes 1–3) as a positive control, and GST (lane 7) as a negative control. Aurora B WT was also incubated with 2 μM of ZM447439 as a specificity control (lanes 3 and 6). Samples were transferred to a filter, stained by Ponceau S (lower panel) and analyzed by autoradiography (upper panel). *, degradation product. Data are reproducible in 3 independent experiments. (B) HeLa cells were synchronized by an overnight incubation with 100 ng/ml nocodazole (M, mitotic) as indicated. Cells (1 X 106) were labeled with 32P orthophosphate for 4 h in the presence or absence of 2 μM ZM447439 (ZM). Cell lysates (300 μg at 1 mg/ml) were immunoprecipitated for NudC, transferred to a filter, analyzed by autoradiography, and immunoblotted for NudC. 32P-NudC was quantified as 32P-NudC/total immunoprecipitated NudC and normalized against NudC signals in asynchronously cycling (Asy) cells.