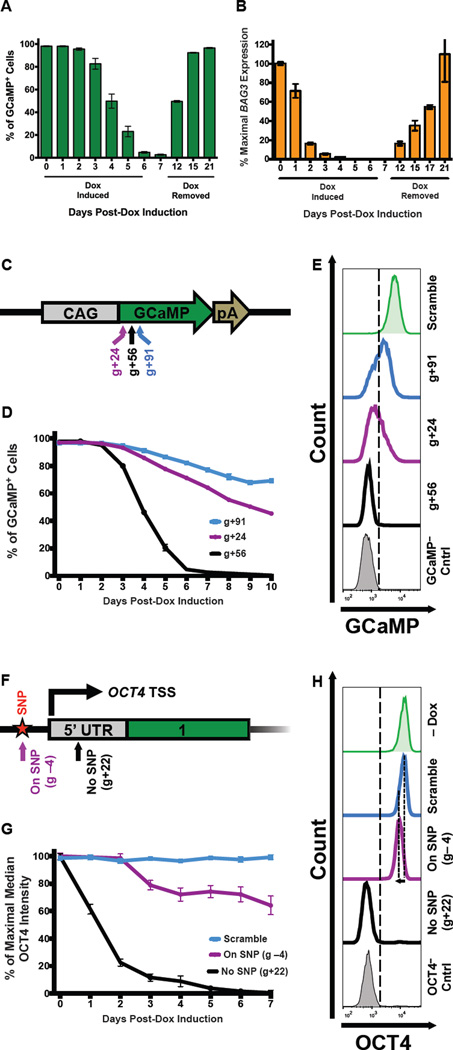
Figure 4. CRISPRi knockdown is reversible and tunable.
A CRISPRi clone containing gRNA against the GCaMP transgene (GCaMP g+56) and endogenous BAG3 locus were used to test the knockdown efficiency and reversibility of the CRISPRi system in iPSCs. (A) Flow cytometry analysis of GCaMP expression showed that after 7 days of doxycycline induction, GCaMP was knocked down by ~99%, and was completely restored after doxycycline withdrawal for 14 days. (B) Using TaqMan qPCR, BAG3 transcript levels were knocked down to nearly undetectable levels, and expression was restored after doxycycline withdrawal. (C) Schematic diagram of the GCaMP-expression vector in which the GCaMP open reading frame (ORF) is driven off the CAG promoter. The locations of three gRNAs (g+24, g+56, and g+91) are schematically highlighted on the GCaMP ORF. The coordinates of GCaMP gRNA are based on the translation start site. pA, poly A signal. (D) Three stable CRISPRi colonies, each containing a different gRNA against GCaMP, were selected using blasticidin and cultured with doxycycline for 10 days. The percentage of GCaMP-positive cells for each gRNA-containing clone was plotted as a function of time based on flow cytometry analysis. Variable levels of GCaMP knockdown (~30%, ~50%, and ~99%) were achieved with different gRNA sequences. Error bars = standard deviation. N=1–3 technical replicates for each time point. (E) Flow cytometry plots of GCaMP fluorescence of stable CRISPRi clones on day 10 of doxycycline treatment. Using different gRNAs that target near the same region, variable levels of knockdown can be achieved. A scramble gRNA-containing CRISPRi and a GCaMP-negative iPSC population are displayed as controls. (F) Partial schematic diagram of the OCT4 locus marked with the location of the TSS and two gRNA-binding locations. Asterisk, a single-nucleotide polymorphism (SNP); green box, exon 1; grey box, 5’ untranslated region (5’ UTR). (G) Three stable CRISPRi colonies, two with different gRNAs against OCT4 and one with a scrambled control, were selected with blasticidin. Stable clones that contain either a scramble gRNA, a gRNA that targets a PAM sequence containing a SNP (OCT4 g−4), or a gRNA that does not target a SNP (OCT4 g+22) were treated with doxycycline. The percentage of the maximal median intensity of OCT4 staining for each gRNA-containing clone is plotted as a function of time by flow cytometry analysis. Complete loss of OCT4 expression (>98% knockdown) was observed after 7 days of doxycycline induction only when both alleles were targeted using OCT4 g+22. While using OCT4 g−4, which targets only one OCT4 allele (due to SNP in the PAM sequence), a gradual loss of OCT4 staining intensity is observed over time (down by ~40% by day 7). Error bars = standard deviation. N=1–3 technical replicates for each time point. (H) Flow cytometry plots of OCT4 staining on day 7 of doxycycline treatment. Dashed lines highlight the loss of OCT4-staining intensity (~40%) when using OCT4 g−4 compared to the scramble control. By targeting only one allele of OCT4, the OCT4-staining intensity homogeneously shifts (while remaining OCT4-positive), indicating that each cell experiences approximately the same level of knockdown. Note that the x-axis is a log-scale of OCT4 intensity. Differentiated iPSC-derived fibroblasts (OCT4− Cntrl) and a non-doxycycline-treated (–Dox) sample are displayed as controls.