Figure 1.
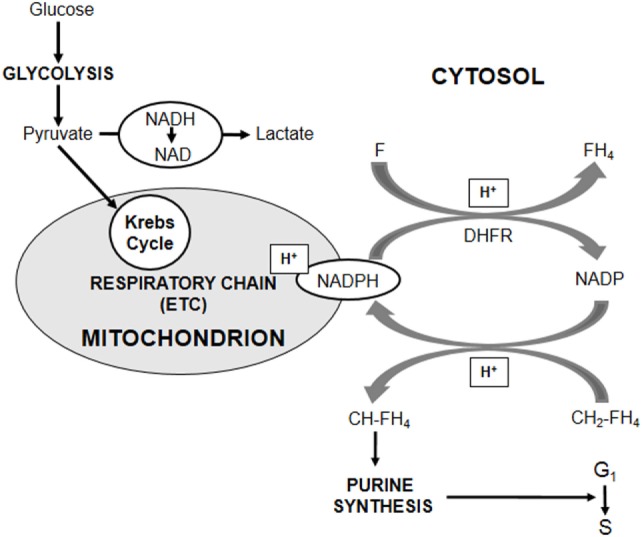
Role of cellular Redox state in the control of cell cycling. The core of the metabolic network controlling AH130 hepatoma cell cycling is the cellular RedOx state expressed by the cytosolic NADP/NADPH ratio. The transfer of reducing equivalents (H+) from methylene-tetrahydro-folate (CH2-FH4) to NADP, generating methenyl-tetrahydro-folate (CH-FH4) and NADPH, is a limiting step of the assembly of purine ring required for the amplification of purine pools indispensable for the G1–S transition of mitotic cycle. An accumulation of cytosolic NADPH inhibits cell recruitment into S. A fundamental role in the regulation of NADP/NADPH ratio is played by folate (F), whose reduction to tetrahydro-folate (FH4) by dehydrofolate-reductase (DHFR) generates NADP. When DHFR activity is impaired by the addition of its inhibitor Methotrexate or of an excess of the reaction product (FH4), NADPH increases with the consequent reductive shift of NADP/NADPH ratio and the inhibition of purine synthesis. However, the major antagonist of this shift is the transfer of cytosolic reducing equivalents onto the mitochondrial ETC through suitable shuttles, accounting for the crucial role of ETC in purine synthesis. This transfer is antagonized whenever ETC, although not inhibited, is saturated by reducing equivalents produced by oxidizable substrates of the Krebs cycle, such as pyruvate.
