FIGURE 5.
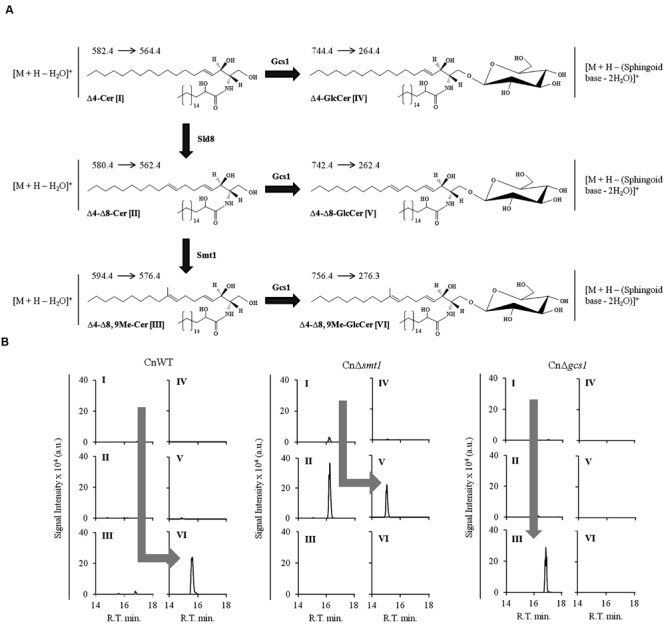
Analysis of ceramides and glucosylceramides of Cryptococcus neoformans using MRM approach. (A) Partial pathway and structures of glucosylceramide biosynthetic pathway of C. neoformans. Δ4-Cer [I] is converted to Δ4, Δ8-Cer [II] by Δ8-desaturase (Sld8). Δ4–8-Cer is then methylated at C9 position of sphingoid backbone to from Δ4–Δ8,9Me-Cer [III] by Smt1. A Gcs1 transfers the glucose group to sphingoid backbone of ceramide structures at C1 position forming a β1-3-glycosidic bond. This results in formation of Δ4-GlcCer (IV), Δ4, Δ8-GlcCer (V) and Δ4–Δ8,9Me-GlcCer (VI) structures. The amide-linked fatty acyl in these structures is α-hydroxylated, a key feature. The respective SRM transitions used for the identification and quantification of these structures are shown as (Parent ion→Daughter ion). (B) Analysis of ceramide and glucosylcamides in sphingolipid pathway mutants of C. neoformans. The wild type strain CnWT shows a major pool of Δ4–Δ8,9Me-GlcCer (VI), where all other structures are present in negligible amounts. The CnΔsmt1 strain does not produce Δ4–Δ8,9Me-GlcCer (VI) structure and accumulates Δ4, Δ8-Cer [II] which is converted to Δ4, Δ8-GlcCer [IV] by Gcs1. The CnΔgcs1 shows an accumulation of Δ4–Δ8,9Me-Cer (III). The pathway trends are represented by gray arrows in the wild type and mutant strains.
