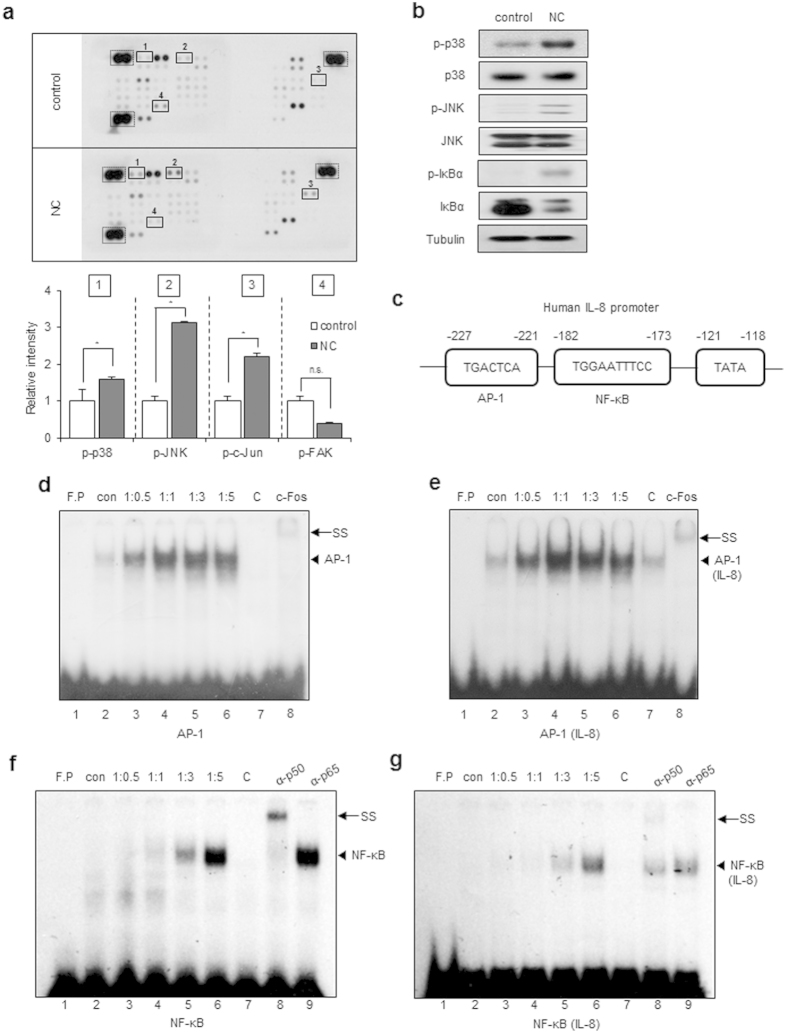
Figure 3. Necrotic cells induce phosphorylation of p38, JNK, and IκBα and IL-8 induction.
(a) CRT-MG cells were either untreated (control) or treated with necrotic cells (NC) for 24 h, and 430 μg of cell lysate were used for the phosphokinase assay as indicated in Methods. The duplicate spots corresponding to the increased phosphorylation of p38, JNK, and c-Jun and decreased phosphorylation of FAK are highlighted in the solid box (left). The dashed line box indicates reference spots. The intensity of spots corresponding to p38, JNK, c-Jun, FAK, and the positive control was quantified using Image J software and subtracted from the background, then expressed as a ratio to the positive control. *P < 0.05 vs. each control. n.s., not significant. (b) Cell lysates from the CRT-MG cells treated with necrotic cells for 24 h were analyzed by immunoblotting to confirm the phosphokinase array results. Tubulin was used as the loading control. (c) The human IL-8 promoter AP-1 and NF-κB binding elements are indicated. (d–g) The nuclear extracts (NEs) from CRT-MG cells treated with or without necrotic cells were incubated with a radiolabeled DNA probe for the human IL-8 promoter and consensus oligonucleotides and subjected to an electro mobility shift assay. NEs were analyzed with probe for either AP-1 consensus oligonucleotide (d), AP-1 sequence within the IL-8 promoter (e), NF-κB consensus oligonucleotide (f), or NF-κB sequence within the IL-8 promoter (g). The competition assay was performed by adding a 100-fold molar excess of cold probe. Anti-c-Fos, p50 or -p65 antibodies were added to test the specificity of interaction. Data shown represent at least three experiments. F.P, free probe; C, competition; SS, supershift.