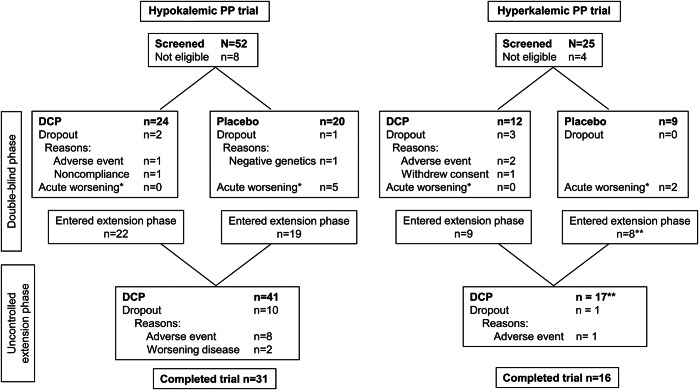
Figure. Flow diagram.
*Participants who reached the endpoint of acute worsening during the double-blind phase had an early week 9 visit and moved directly into the 52-week uncontrolled extension phase. **Data from one participant in the hyperkalemic periodic paralysis (PP) trial were included in the 9-week phase, but not in the 52-week uncontrolled extension phase as the participant had been randomized to acetazolamide in this phase (the trial was initially designed to include a comparison against acetazolamide; however, the acetazolamide arm had to be abandoned owing to poor recruitment). Forty-five participants were randomized to the hypokalemic PP (HOP) trial, but data from one participant assigned to receive dichlorphenamide (DCP) were not used because the participant did not meet diagnostic criteria for HOP (this participant is not shown in this figure). The decision to not include the participant was made prior to unblinding. One participant in the HOP trial assigned to receive placebo in the double-blind phase was mistakenly treated with placebo during the extension phase; data from this participant were not included in the analyses of data from the extension phase.