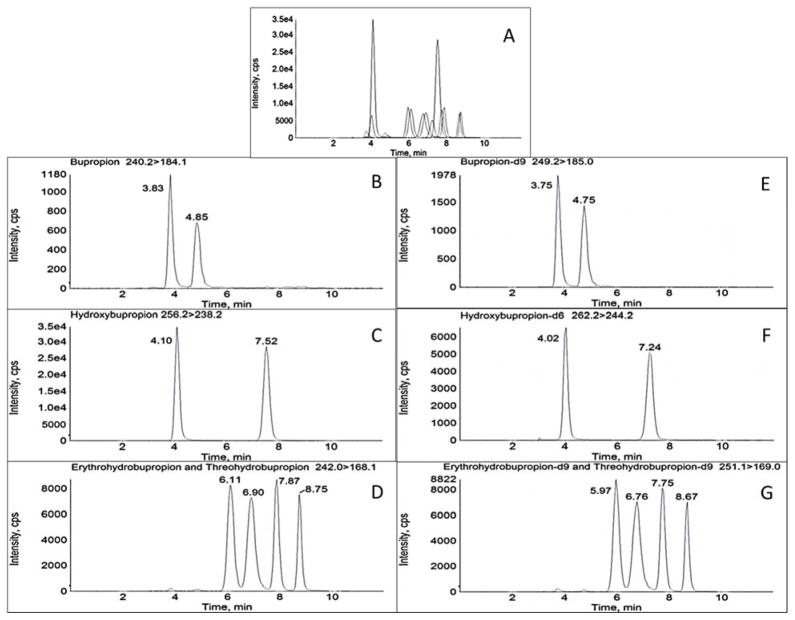
Figure 2.
Chromatographic separation of bupropion, hydroxybupropion, erythrohydrobupropion, and threohydrobupropion enantiomers and respective deuterated standards in processed plasma on an AB Sciex 3200 mass spectrometer. Numbers in parenthesis refer to retention times in min. A: Total ion chromatogram. B–G: Representative MRM chromatograms B: (R)-bupropion (3.83), (S)-bupropion (4.85). C: (R,R)-hydroxybupropion (7.52), (S,S)-hydroxybupropion (4.10). D: (1S,2S)-threohydrobupropion (6.11), (1R,2R)-threohydrobupropion) (6.90), (1R,2S)-erythrohydrobupropion (7.87), and (1S,2R)-erythrohydrobupropion (8.75). E: (R)-bupropion-d9 (3.75), (S)-bupropion-d9 (4.75). F: (R,R)-hydroxybupropion-d6 (7.24), (S,S)-hydroxybupropion-d6 (4.02). G: (1S,2S)-threohydrobupropion-d9 (5.97), (1R,2R)-threohydrobupropion-d9) (6.76), (1R,2S)-erythrohydrobupropion-d9 (7.75), and (1S,2R)-erythrohydrobupropion-d9 (8.67). Concentrations correspond to Calibration level 6 (10 ng/mL R- or S-bupropion; 250 ng/mL (R,R)- or (S,S)-hydroxybupropion; 50 ng/mL (1R,2S)- or (1S,2R)-erythrohydrobupropion; 50 ng/mL (1S,2S)- or (1R,2R)-threohydrobupropion)