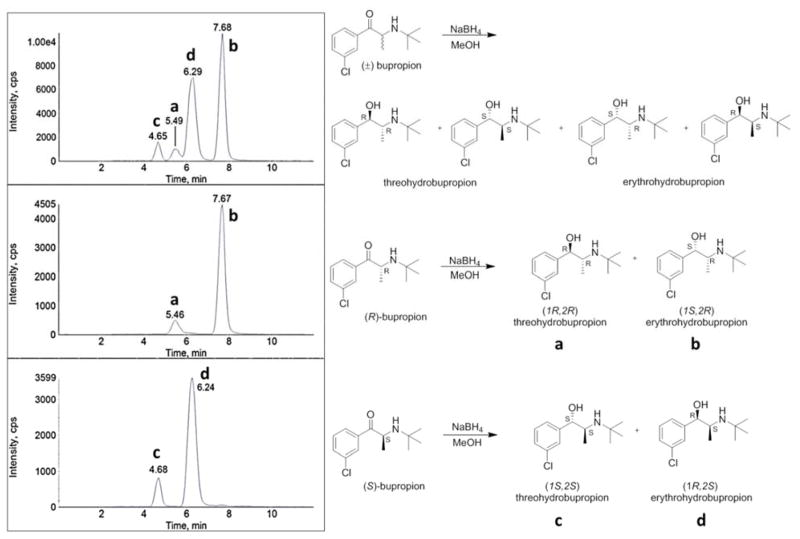
Figure 3.
Identification of erythrohydrobupropion enantiomer peaks and threohydrobupropion enantiomer peaks. Representative MRM chromatograms from the sodium borohydride reduction of: A: racemic (R,S)-bupropion, B: (R)-bupropion, and C: (S)-bupropion. Experiments were performed utilizing an AB Sciex 3200 instrument. Threohydrobupropion enantiomers elute at 4.65 (c) and 5.49 (a) min, respectively. Erythrohydrobupropion enantiomers elute at 6.29 (d) and 7.68 (b) min, respectively. To accurately identify each of the enantiomers, retention times of the peaks in panel A (a–d) were aligned with the retention times of the peaks in panel B (a and b) and C (c and d), respectively.