ABSTRACT
Hepatitis B virus (HBV) is susceptible to the cellular immune responses, especially to the signal of interferon (IFN)-γ. The action of IFN-γ is pleiotropic, and causes downregulation of HBV in protein, RNA, and possibly DNA levels. Therefore, therapeutic vaccination to induce cellular immune responses to HBV is a promising approach for controlling chronic HBV infection. A number of clinical trials with this approach have been conducted to date, however, they have not been as successful as initially expected. T-cell exhaustion induced by the excessive HBV antigens caused by persistent infection is thought to be one of the main causes of poor responses to therapeutic vaccination. In this review, the mechanisms behind immunoregulation of HBV replication and immunodysfunction during chronic HBV infection are summarized, and novel approaches to improve the efficacy of therapeutic vaccination, from basic research to clinical trials, are introduced.
Key Words: chronic hepatitis B, interferon-γ, adaptive immune response, therapeutic vaccination, T-cell exhaustion
INTRODUCTION
Hepatitis B virus (HBV) is a type of the hepadnavirus which is spread by contact with infected blood and body fluids, and causes acute and chronic necroinflammatory liver diseases.1-3) Since HBV is a noncytopathic virus, inflammation in the liver is mediated by host immune responses to the HBV-infected hepatocytes. HBV infection in immunocompetent adults results in a self-limited, transient liver disease, and subsequent viral clearance is achieved in more than 95% of adults, whereas more than 90% of neonates exposed to HBV at birth become persistently infected.1-3) Even in persistently infected individuals, hepatitis B e antigen (HBeAg)/antibody (Ab) seroconversion (SC) with marked reduction of HBV replication, associated with biochemical and histologic regression of liver inflammation, occurs in the majority of patients in their natural course. If a reduction of HBV replication does not occur, the infected individuals are under a greater risk of developing cirrhosis (LC), liver failure, and hepatocellular carcinoma (HCC).1-3)
Chronic liver diseases associated with chronic HBV infection are serious public health problems worldwide. It is estimated that 370 million people are chronically infected with HBV, and that up to 1.2 million people die every year due to the complications of HBV-related chronic liver diseases, such as LC and HCC.4-6) HBV infection has been the most significant factor associated with the development of liver cancer, which is one of the most malignant cancers; the second most frequent cause of cancer death in men, and the sixth leading cause of cancer death in women.4-6) HBV infection accounts for about 60% of all HCC occurrences in developing countries and about 23% of cancer occurrences in developed countries; the corresponding percentages for hepatitis C virus (HCV) infection are 33% in developing countries and 20% in developed countries.6) As of 2008, a total of 177 countries (91%) had introduced the HBV vaccine for their national infant immunization schedules.7,8) This efficient vaccination method decreases the incidence of HBV infection; however, chronic HBV infection remains a serious problem in countries with a higher prevalence of chronic HBV infection, such as those in Asia and sub-Saharan Africa, where the prevalence exceeds 8% of their populations.6,9) Even in Japan, where the HBV carrier rate is about 1% and is gradually decreasing, thanks to the success of a national program for immunoprophylaxis of perinatal HBV transmission, the number of HBV-related HCC is not decreasing10,11). It is obvious that there is an urgent need for the effective treatment control and termination of HBV infection.
It is considered that permanent and profound suppression of viral replication is beneficial for preventing complications of chronic hepatitis B (CHB), as persistent HBV viremia has been proven to be the most important predictor of progression to LC, hepatic failure, and development of HCC.12) Therefore, the initial goal of the treatment is to suppress active viral replication and to subsequently restrain the activity of hepatitis.
Currently, CHB patients are treated mainly with antiviral reagents, i.e., nucleoside or nucleotide analogues (NAs). NAs target the reverse transcriptase of HBV and are potent inhibitors of viral replication. NA treatment usually results in a rapid decline of serum HBV DNA levels, and long-term therapy results in reduction in hepatic fibrosis, hepatic decompensation, and liver-related mortality.13-15) Induction of NAs opened a new era in the treatment of CHB, providing a safe, effective and well-tolerated therapeutic option. However, at the same time, there is a drawback of NA treatment, which is not negligible. Since the complete eradication of HBV infection is rarely achieved with this treatment, NA must be administered for an extremely long period of time. And long-term treatment is occasionally associated with an increased risk for development of viral resistance to the drugs, which eventually results in recurrence and progression of the disease.16-18)
Interferon (IFN)-α/β is another option for CHB treatment. It has a direct antiviral effect, but is thought to act mainly by augmenting host immune response specific to the HBV-related antigens.15,19,20) Because of its immunomodulatory mechanism of action, IFN-α/β requires only a limited treatment period, and brings out a durable response even after the discontinuation of the treatment if the endpoint response is favorable.21) In addition, recently approved pegylated IFN (Peg-IFN)-α shows a slightly increased efficacy compared to standard IFN-α/β.15,22-25) However, the response rate by IFN therapy is not necessarily high, despite the use of Peg-IFN-α (sustained response rate: 10 to 30%),15,22-25) and IFN-based therapy is associated with a wide spectrum of adverse events, including flu-like symptoms, decrease of white blood cells and platelets, and depression. In addition, IFN is contraindicated in patients with decompensated LC.
As seen in the case of IFN treatment, the key factor for obtaining permanent control over HBV is to induce effective virus-specific immune responses which are strong enough and effectively regulate viral replication. In other words, therapeutic vaccination is a promising candidate for the treatment of chronic HBV infection.
Currently, the only available vaccine for HBV is the hepatitis B surface antigen (HBsAg) vaccine, which is usually used for the prophylaxis of HBV infection, as mentioned above. The HBsAg vaccine elicits a level of anti-hepatitis B surface antigen antibody (HBsAb) high enough to protect against the infection of newly intruding HBV in more than 90% of vaccinees, if properly administered.26) However, therapeutic use of this HBsAg vaccine has not brought satisfactory results for controlling viral replication at present.27.28) Therefore, a new vaccine or novel strategy is definitely needed to achieve sustained control of chronic HBV infection.
In this review, the mechanisms of immune responses that control HBV replication, their application to therapy, and current and future approaches for developing a vaccination strategy to achieve sustained control or eradication of HBV, are described.
Immune responses that control HBV replication
In general, the immune responses that terminate viral infection work in several steps. During the early phases of acute viral infection, natural killer (NK) cells and natural killer T-(NKT) cells are the first lines of defense, and the activation of these cells helps to reduce the viral load through the secretion cytokines, such as IFN-γ.29-32) In the liver, the frequencies of NK and NKT cells are higher than in other organs,33) and these cells mediating innate immunity are thought to play certain roles in the pathogenesis of HBV infection. Indeed, during the early phases of acute HBV infection, the activation of NK cells helps to reduce the viral load through the action of IFN-γ.34) However, this activation is rapidly suppressed by interleukin (IL)-10 at the peak of viremia, indicating that the roles of NK cells on HBV regulation are rather limited.35) In fact, in chimpanzees infected by HBV, the innate immune cells, such as NK and NKT cells, do not significantly contribute to the pathogenesis of viral hepatitis during the symptomatic phase of acute or chronic HBV infection.36) Therefore, it is assumed that a crucial role in the pathogenesis of HBV infection and the control of HBV replication is played by the adaptive immune response primed after the initial response by the innate immunity.
HBV transgenic mouse hepatitis model for analysis of liver pathogenesis
The roles of specific immune responses on the pathogenesis of HBV infection and viral clearance have been well examined in the hepatitis model with HBV transgenic mouse (Fig. 1). The adoptive transfer of HBV-specific cytotoxic T-lymphocyte (CTL) lines and clones into immunologically tolerant HBV transgenic mice produced liver disease with hepatocyte necrosis and inflammation that is histologically very similar to acute viral hepatitis in human.1,3, 37-43)
Fig. 1.
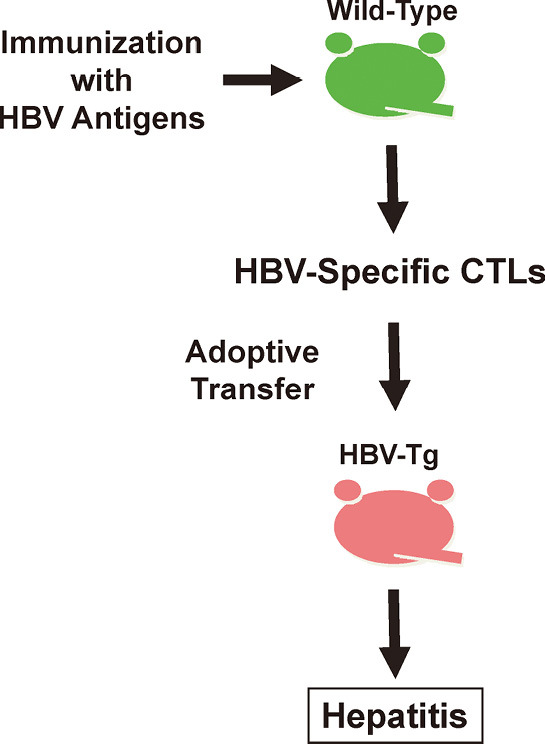
HBV transgenic mice hepatitis model. Nontransgenic (B10.D2; H-2d) mice were immunized with either recombinant vaccinia virus or HBsAg protein. Spleens were harvested and stimulated in vitro with irradiated HBsAg-expressing P815 cells. Primed spleen cells were restimulated and expanded in vitro, and cloned by limiting dilution method. HBsAg-specific CTL clones or cell lines were intravenously injected to HBV transgenic mice (1.3.32; inbred B10.D2, H-2d). Liver inflammation and viral clearance were observed in HBV transgenic mice.
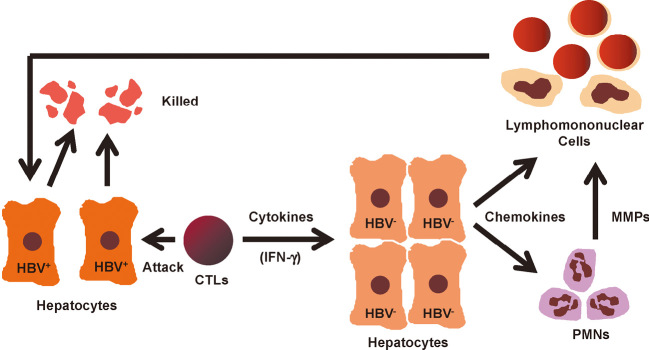
The initial step in the disease process after adoptive transfer is apoptosis caused by the direct attack of CTLs against viral antigen-positive hepatocytes. Apoptosis occurs immediately after the transfer of CTLs to mice and is necessary for further steps in the disease process. The direct CTL-target cell interaction results in the appearance of widely scattered apoptotic hepatocytes.37-39,42) After the initial step of direct CTL killing, many host-derived inflammatory cells, such as polymorphocnuclear neutrophils (PMNs), antigen-nonspecific T-cells, NK cells, and macrophages, are recruited around apoptotic hepatocytes and virus-specific CTLs, and form necroinflammatory foci, which resembles the histology observed in acute hepatitis in men.37-39,42) This process, from hepatocyte apoptosis to necroinflammatory foci, is shown to be associated with the action of activated platelets,43) cytokines such as IFN-γ and the tumor necrosis factor (TNF)-α,37,40,41) chemokines such as CXCL9 and CXCL10,44) and matrix metalloproteinases (MMPs) produced by PMNs (Fig. 2).45)
Fig. 2.
Mechanisms of CTL-induced liver disease and viral clearance. HBV-specific CTLs kill antigen-expressing hepatocytes via Fas lingad- and perforin-mediated pathways, and produce antiviral cytokines, such as IFN-γ, that inhibit HBV replication noncytopathically in a greater number of adjacent cells. IFN-γ activates hepatpcytes to produce chemokines that recruit antigen-nonspecific polymorphonuclear cells (PMNs) and antigen-nonspecific lymphomononuclear cells (e.g., NK cells, T-cells, and macrophages) into the liver. Production of matrix metalloproteinases (MMPs) by PMNs also promotes migration of antigen-nonspecific lymphomononuclear cells. These antigen-nonspecific inflammatory cells amplify liver disease initiated by CTLs. The figure is modified from that of ref #3.
Mechanisms of HBV elimination by adaptive immune response
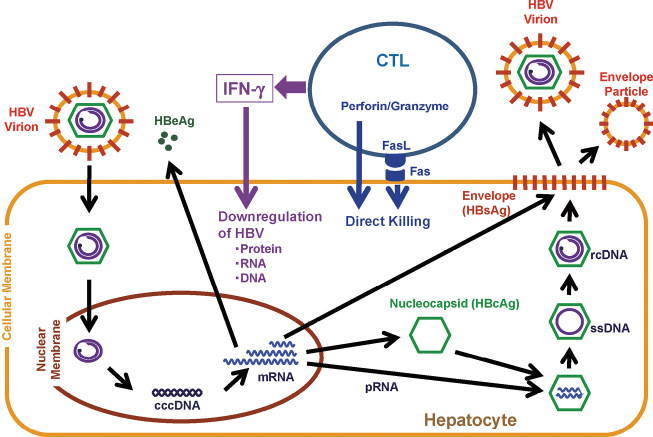
In the same HBV transgenic mouse model, the mechanisms of viral elimination by adaptive immune response is examined in detail. As mentioned above, HBV-specific CTLs directly kill HBV-expressing hepatocytes, and the recruited host-derived inflammatory cells also cause damage to infected hepatocytes, thus reducing the viral load to some extent (Fig. 2). However, more importantly, IFN-γ produced by activated CTLs following antigen recognition is responsible for most of the antiviral potential of the CTL-mediated immunity.40,41,47) The action of IFN-γ on HBV elimination is thought to be pleiotropic. It is indicated that IFN-γ hinders the assembly of pregenomic HBV RNA-containing nucleocapsid protein in a proteasome- and kinase-dependent manner.48-50) Also, IFN-γ causes destabilization of HBV RNAs in the nucleus by a La-dependent mechanism. Briefly, HBV RNAs are stabilized in the nucleus by the binding of the full length of La, which is known as an autoantigen of Sjögren’s syndrome. However, IFN-γ produced by activated CTLs induces proteolytic cleavage of La, and the subsequent binding of cleaved La to HBV RNAs results in the destabilization of HBV RNAs, i.e., facilitating the destruction of HBV RNAs by nuclear RNases.51-55) IFN-γ also effectively downregulates replication intermediates of HBV DNA in a nitric oxide (NO)-dependent manner, while the effect of IFN-γ on covalently closed circular DNA (cccDNA) in the nucleus is not yet clarified (Fig. 3).36,56,57) Thus, with all these mechanisms, CTLs and IFN-γ produced by CTLs bring about a very profound downregulation of HBV, most of which is accomplished without destroying hepatocytes.
Fig. 3.
HBV life cycle and CTL-mediated viral clearance. The process of HBV entry into hepatocyte is poorly understood, however, it is thought to be a receptor-mediated process. Once the virion enters the cytoplasm, it is uncoated; the uncovered nucleocapsid is transported to the nucleus. Following nucleocapsid disassembly, the second strand of the open circular viral genome is completed, and the ends of each strand are ligated. This process results in production of a covalently closed circular DNA (cccDNA), which is the transcriptional template of the virus. Transcription by viral polymerase leads to production of viral RNAs which are transported from the nucleus to the cytoplasm. Transcripts are translated into corresponding proteins, such as envelope proteins, nucleocapsid proteins, HBeAg, polymerase protein, and X protein. The envelope proteins traverse the ER membrane as integral membrane proteins. HBcAg and polymerase proteins assemble around pregenomic RNA (pRNA) to form HBV RNA-containing nucleocapsid, within which RNA is reverse transcribed to produce the first single-strand viral DNA (ssDNA). ssDNA serves as the template for second-strand DNA synthesis, producing nucleocapsid containing a partially double-stranded, relaxed circular DNA molecule (rcDNA). At the ER membrane, it interacts with the envelope proteins that trigger an internal budding reaction. This reaction results in the formation of virion which is transported out of the cell. HBV-specific CTLs recognize and cause death to the HBV-infected cells via Fas lingad- and perforin/granzyme-induced pathways. Activated CTLs produce cytokines, such as IFN-γ, which causes profound, pleiotropic downregulation of the virus noncytopathically in adjacent infected cells.
Helper T-cells (Ths) also have the potential to eliminate HBV, since they are important producers of IFN-γ. In the HBV transgenic mouse model, it was indicated that HBV-specific Ths also cause HBV downregulation in an IFN-γ-dependent manner, but less efficiently, compared to CTLs.38) On the other hand, Ths are also thought to contribute to controlling HBV infection by assisting in the induction and maintenance of HBV-specific CTLs by regulating immune response.38) Indeed, strong HBV-specific Th responses are usually associated with significant CTL responses in humans and chimpanzees that resolve HBV infection.1,59)
Considering the mechanisms of HBV elimination by adaptive immune responses, it is obvious that therapeutic vaccination that induces HBV-specific CTLs and Ths has great potential as a strategy to terminate chronic HBV infection.
Alteration of immune response during chronic HBV infection
Despite the presence of immunological mechanisms of HBV elimination, the virus is rarely eradicated when the infection becomes chronic. The key features of chronicity of HBV infection are the impairment of immune responses related to the cellular composition of the liver, and the presence of excessive viral antigens caused by persistent infection.
The liver is composed of parenchymal hepatocytes and non-parenchymal cells, including biliary epithelial cells (BECs), hepatic stellate cells (HSCs), sinusoidal endothelial cells (LSECs), and Kupffer cells (KCs). Importantly, most of these cells do not express enough costimulatory molecules to induce functional activation of antigen-specific T-cells. Therefore, HBV antigen presentation by these cells tends to lead T-cells toward tolerance rather than activation.60-62)
Unlike the cell populations mentioned above, dendritic cells (DCs) professional antigen-presenting cells (APCs) do express costimulatory molecules, and play an important role in the induction of functional T-cells. However, in patients with CHB, decreases in the number of circulating dentritic cells (DCs) and functional impairment of both myeloid (mDCs) and plasmacytoid DCs (pDCs) have been reported, and they may account for the dysfunction of the adaptive immunity.63-65)
Chronic exposure to excessive antigens leads T-cells to progressive exhaustion, when they lose the ability to differentiate into memory cells and the effector function, e.g., proliferative capacity, cytotoxicity, and cytokine production (IFN-γ, TNF, etc.).66-68) The exhausted T-cells express inhibitory receptors on their surface, such as programmed death-1 (PD-1), and the interaction between PD-1 and its ligand, PD-1 ligand 1 (PD-L1) are thought to be the major inhibitory receptor pathways involved in T-cell exhaustion.66-68) Indeed, PD-1 is shown to be overexpressed on HBV-specific T-cells, which produce only a small amount of IFN-γ.67,69) As T-cell exhaustion progresses along with prolonged exposure to high doses of antigens, it may finally result in the clonal deletion of HBV-specific T-cell populations. In addition, regulatory Foxp3+CD4+ T-cells (Tregs) are known to affect immune responses during chronic infections, and are thought to affect the exhaustion of antigen-specific T-cells.67) However, the role of Tregs in chronic HBV infection is not clearly understood.70-73)
Even though our understanding of the role and alteration of immune responses during chronic HBV infection has been greatly improved, many questions remain to be elucidated. It is important to continue attempting to answer these questions in order to develop new strategies of therapeutic vaccination.
Prophylactic HBsAg vaccines for therapeutic vaccination
The first series of therapeutic vaccine trials were conducted with commercially available prophylactic HBsAg vaccines.27,74-77) An initial pilot study demonstrated that HBsAg-vaccination induced cell-proliferated responses and IFN-γ production specific for HBsAg, and reduction of serum HBV DNA levels in some patients (7 out of 27 patients were positive for proliferative response to envelope protein).27) Further studies also proved that HBsAg vaccination induced HBsAg-specific T-ell responses, a decrease of HBV DNA levels, and a significant increase in HBeAg/antibody (Ab) seroconversion (SC); however, most of these effects were temporal.76,77) In addition, only a few patients showed HBsAg clearance and development of antibody against HBsAg in these studies.76,77) Since the ultimate goal of CHB treatment is the clearance of HBsAg and the induction of HBsAb, which can neutralize viral particles, the use of HBsAg for therapeutic vaccination seems to be a reasonable choice. However, the results of these studies clearly indicate that the classical prophylactic vaccines do not have enough antiviral potential, and that development of novel vaccines or a strategy with greater efficacy is necessary.
Studies also showed that HBsAg vaccines are more effective when used in patients with low viral loads, suggesting the possibility that the presence of excessive viral antigens lessens the efficacy of vaccination. Therefore, combining antiviral drugs that can suppress viral replication and reduce the viral antigen load simultaneously through the vaccination is thought to be one of the better therapeutic approaches.
Combining antiviral drugs to vaccination
Lamivudine (LMV) is the first NA used for the treatment of HBV infection.78,79) LMV itself does not have a direct immunomodulatory effect, however, LMV treatment is shown to restore the responses of HBV-specific T-cells during the first few months of the treatment period, and other NAs may have the same effect. This means that reducing the HBV antigen load by antiviral therapy itself may increase the responsiveness of HBV-specific T-cells,80) probably by restoring the function of exhausted T-cells. Since the restoration of T-cell responses is weak, transient, and does not lead to an increase of sustained viral response, such as HBeAg/Ab SC,81) there have been a number of attempts to combine NA administration to vaccination to gain continuous activation of HBV-specific T-cell responses (Fig. 4).
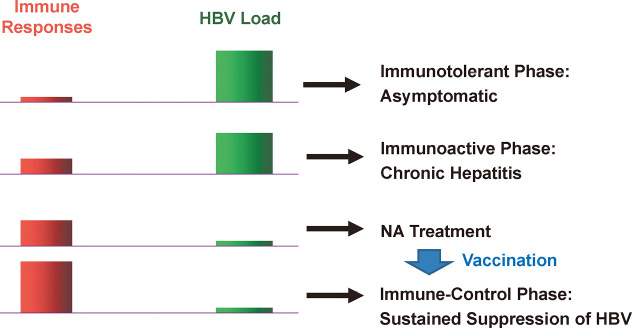
Fig. 4.
Balance between immune response and viral load in chronic HBV infection. In patients with perinatally acquired HBV infection, immunotolerant phase is the first phase of infection, when patients are asymptomatic without significant immune responses and with higher viral load. The second phase is the immunoactive phase, when patients have liver inflammation with slight increase of immune responses and slight decrease of viral load. If spontaneous HBeAg/Ab seroconversion (SC) does not occur, liver damage progresses from chronic hepatitis to cirrhosis. Nucleoside or nucleotide analogue (NA) treatment can reduce viral load and is thought to increase immune responses probably by restoring the function of exhausted T-cells. Since increase of immune responses is partial and transient, therapeutic vaccination to increase immune responses is recognized as a promising measure to achieve sustained control of HBV replication.
Combining LMV to vaccination is the most widely applied method of treatment, and is found to be well tolerated and safe, without serious adverse effects. This combination therapy is reported to give a significantly higher and earlier rate of viral suppression than LMV or vaccine monotherapy, and increases the rate of HBeAg/Ab SC to some extent.82-84)
We also conducted a controlled trial composed of 53 patients with CHB, 33 with HBeAg+, and 20 with HBeAg–, in which participants randomly received either LMV monotherapy or the combination therapy of LMV and HBsAg vaccine. In the HBeAg– group, LMV treatment by itself was fairly effective, and no difference was seen with regard to HBV DNA levels, the rate and period of complicating viral breakthrough (BT), or breakthrough hepatitis (BTH) between the two groups. In the HBeAg+ group, the decrease of serum HBV DNA was faster and the rate of HBV DNA negativation was higher in the combination therapy group, while the ratio of HBeAg/Ab SC did not differ between the two groups. Also, in HBeAg+ patients, the rates of developing BT and BTH were significantly lower in the combination therapy group than in the monotherapy group. Furthermore, there was a tendency for the time period before developing BT to be longer in the combination therapy group than in the monotherapy group. However, most of the patients in both groups developed post-treatment relapse after the cessation of LMV in both groups.85)
Type I IFN is used as an antiviral drug in the treatment of HBV infection, but has the potential to enhance HBV-specific immunity, as mentioned. In general, it enhances both T- and B-cell response by upregulating the expression of human leukocyte antigen (HLA) class I on the surface of APCs. It thereby promotes CTL responses by augmenting and maintaining the production of IgG from plasma cells.19,20) Indeed, in haemodialysis patients whose immune responses were impaired, combining IFN-α to a prophylactic HBsAg vaccine was reported to improve the efficiency of the vaccination.86) There are a few reports describing the efficacy of the combination therapy of type I IFN and HBsAg vaccine for the treatment of CHB. In these reports, combination therapy was reported to accelerate the reduction of HBV DNA and to improve the HBeAg/Ab SC rate compared to IFN monotherapy, but without significant differences.87,88)
In the studies of therapeutic vaccination combined with either NA or IFN, there is a difficulty when generalizing the obtained results, since treatment protocols, backgrounds of the subjects, and the criteria for defining virological responders vary so much. In addition, only a few reports have analyzed cellular or humoral immune responses specific to HBV, and no obvious association was found between HBsAg-specific T-cell responses and any clinical or virological parameters, such as HBeAg/Ab SC and HBsAg clearance, in most of the studies. The protocol of treatment should be deliberately reconsidered, and the immunological analysis should be carefully planned for further improvement of its efficacy.
Recently, an in vitro study demonstrated that the 50-end triphosphate hepatitis B virus X gene (HBx)-short interfering RNAs (siRNAs) exerted significantly stronger inhibitory effects on HBV replication, with a retinoic acid-inducible gene-I (RIG-I) and in a type I IFN-dependent manner. Moreover, its efficacy was confirmed in HBV carrying mice.89) While there is the unsolved problem of how to deliver siRNAs as a drug, they may be candidates for antiviral drugs that can be combined with vaccination as well as NAs.
Use of adjuvants
Adjuvants have been used for decades to improve the immune response to vaccine antigens. The use of adjuvants with vaccine is aimed at enhancing, accelerating, and prolonging the specific immune response towards the desired response to vaccine antigens.90) Therefore, adjuvants are suitable for the purpose of therapeutic vaccination for HBV infection.
The major means by which adjuvants exert themselves are through the upregulation of antigen/adjuvant uptake to the antigen presentation cells (APCs), or the potentiation of immune responses, both quantitatively and qualitatively. Alum, which is used for prophylactic HBsAg vaccine, oil emulsion, and immune stimulating complexes (ISCOMs) (including Quillaia saponins, or liposomes) are used for the former purpose, and monophosphoryl lipid A (MPL) of Toll-like receptor (TLR) 4 agonist, CpG DNA of TLR9 agonist, poly I:C of TLR3 agonist, Montanide-ISA 51, Montanide-ISA 720, MF59, or QS-21 of saponin derivatives, are used for the latter purpose.91) Most of the adjuvants listed above have been tested for their ability to enhance the immune responses to HBV antigens, and have been proven effective. Clinical trials for the application of the vaccines which include some of these adjuvants, mainly for prophylaxis of HBV infection, are in progress or have been completed, such as “HEPLISAV,” comprised of the HBsAg and TLR9 agonist.92) Therapeutic use of the vaccines containing these adjuvants for the treatment of CHB is expected to be effective, however, we must wait for the results of appropriate clinical trials.
There have been attempts to use cytokines, such as IL-2 and IL-7, as adjuvants. IL-2 is a strong Th1-inducing cytokine, and is expected to increase the efficacy of therapeutic vaccination by augmenting Th1 responses that help the induction and maintenance of antigen-specific CTLs. Several trials, including one for HBV infection, have evaluated the efficacy of IL-2 as an adjuvant of therapeutic vaccine.93) However, the use of IL-2 for treatment should be conducted carefully because there is a potential concern that IL-2 administration may increase Tregs, which constitutively express the IL-2 receptor. IL-7 is essential for the homeostatic proliferation of T cells in vivo, and may increase the pool of naïve T-cells potentially stimulated by vaccination.94) A clinical trial combining antiviral treatment with HBsAg vaccine and multiple IL-7 injections is currently underway.
Blocking the PD-1/PD-L1 inhibitory signals on exhausted T-cells by anti-PD-L1 antibodies may be an option for enhancing the efficacy of vaccination. Indeed, it has been suggested that restoration of intrahepatic HBV-specific T-cell responses in CHB patients was possible by blocking PD-1/PD-L1 signals in vitro.69,95)
In the transgenic mouse model, an anti-CD40 agonistic antibody was reported to inhibit HBV replication noncytopathically by a process associated with the recruitment of dendritic cells, macrophages, T-cells, and NK cells into the liver, and the induction of inflammatory cytokines, such as IFN-γ.96) The antibody itself shows an antiviral potential by activating APCs nonspecifically, however, it may also be a candidate of an adjuvant for vaccination, since stimulation of CD40 on DCs and B-cells has been proven to augment cell-mediated and humoral immunity.97)
We have recently demonstrated that alpha-galactosylceramide (α-GalCer), a ligand for NKT cells, significantly enhanced the induction and proliferation of HBsAg-specific CTLs by HBsAg vaccination in the mouse model. Even in HBV transgenic mice which are deeply tolerant to HBV antigens, co-administration of HBsAg and α-GalCer successfully induced HBsAg-specific CTLs.98) Although this approach could not clear HBsAg from transgenic mice despite the induction of HBsAg-specific CTLs, we believe that it is one of the promising approaches to augment the efficacy of vaccination.
Other forms of vaccine candidates
Several types of vaccines with different compositions have been proposed with the aim of increasing vaccination efficacy.
There have been a number of attempts to use peptide-based vaccines targeting T-cell epitopes for the treatment of viral infections as well as cancers, since they are easily produced in large amounts and can be safely administered to patients. A lipopeptide-based vaccine named “Theradigm-HBV,” the HLA-A2.1-restricted, dominant CTL epitope, HBV core Ag (HBcAg)aa18–27, linked to the universal Th epitope tetanus toxoid (TT) has been tested for its ability to induce CTL responses in healthy individuals and CHB patients. “Theradigm-HBV” was capable of inducing HBV-specific CTL responses in a dose-dependent fashion even in CHB patients; however, the clinical effects, such as HBV DNA decrease, were not satisfactory.99,100) While the efficacy of peptide-based vaccine may be further improved with the use of an appropriate adjuvant, the design of the vaccine construct should be carefully considered. Since the problem of HLA restriction is inevitable in peptide-based vaccine, an HLA allele targeted to the binding of epitope peptide should be selected in order to cover a large population of a relevant country or region. In addition, the emergence of vaccine escape variants should be considered, especially in the case of a viral vaccine. We have previously shown that the CTL response to individual viral epitopes can be markedly polyclonal and multispecific, and that mutational inactivation of a single site of the epitope will not usually lead to viral escape.101) Even though a viral mutation which can evade the CTL attack may emerge, simultaneous use of plural epitope peptides may reduce the risk of disabling vaccine efficacy.
HBsAg-HBsAb immune complexes (IC) with alum as the adjuvant have been developed, targeting DCs102). In vitro study has demonstrated that IS stimulated DCs to secrete a large amounts of IL-12, a key cytokine of CD4+ T-cell differentiation into type 1 T helper (Th1) cells that can promote cell-mediated immunity.103) In the phase II trial, a significant virological effect, such as a higher HBeAg/Ab SC rate compared to the control group, was observed in the IC-treated group.102) However, the immune responses that could explain this therapeutic effect, were not analyzed. It makes the evaluation of the efficacy of this vaccine rather difficult, as with other vaccine trials.
It is known that during acute hepatitis, the HBcAg and the polymerase are specific targets of the immune response as well as envelope antigens.1,104) Therefore, the use of a combination of recombinant HBsAg and HBcAg, known as “NASVAC,” is a promising therapeutic vaccine candidate for treating CHB patients. These antigens form virus-like particles, and they work together in the development of cellular and humoral immune responses. In addition, this novel vaccine could be administered in a non-invasive way, i.e., intranasally.105,106) Clinical trials are underway, and the results are awaited.
DNA-based vaccines have been tested for both prophylactic and therapeutic purposes, and encouraging results have been reported in the animal models. The advantage of a DNA vaccine is that it can induce both humoral and cellular immune responses. The phase I trial administering a DNA vaccine which encodes HBV envelope proteins in CHB patients showed favorable results.107) DNA vaccination led to the induction of IFN-γ-secreting T-cells and CTLs specific to envelope antigens, and to an increase in an NK cell subset known to produce abundant cytokines.108) The clinical trials with DNA vaccination are increasing, and most of them have shown promising results. However, these trials should be carefully conducted, since there are concerns about potential side effects, such as possible integration of plasmid DNA into the host genome, that result in mutations.
Few studies have reported the use of autologous DCs pulsed with HBV antigens for therapeutic purposes. One of them is a pilot study with a small number of CHB patients that evaluated the safety of HBsAg-pulsed autologous DCs. The administration of DCs was safe with no exacerbation of liver damage; however, the number of patients was too small to evaluate the efficacy of vaccination.109) Another recent trial was conducted using autologous DCs pulsed with HLA-A2-restricted peptides derived from HBcAg (aa18–27), with re-injection of middle envelope protein in CHB patients.108) The treatment was more effective in the patients with HBeAg– or with a low viral load, and some of them had cleared HBsAg. However, in that study, the immune response-mediated mechanisms accounting for the observed serologic and virological responses were not reported, raising questions about the specificity of this DC-mediated therapy.110)
CONCLUSION
Therapeutic vaccination is a reasonable approach for the treatment of chronic HBV infection, considering the natural course of HBV infection and the susceptibility of HBV to the signal of IFN-γ. And there have been a number of attempts at therapeutic vaccination, including those using conventional prophylactic vaccine, those combining antiviral drugs with vaccine, those using vaccine with novel adjuvant, or those using newly-developed vaccines as introduced in this review (Fig. 5). The efficacies of current therapies are still lower than initially expected. However, the efficacy may be improved thanks to the progress made in understanding the underlying mechanism of immunodysfunction in HBV carriers, and the development of new therapeutic tools for immunostimulation. The efforts to improve the efficacy of vaccine therapy should be continued in order to establish an ideal therapeutic vaccination for the treatment of CHB. While most of these therapeutic vaccines have been safely used without serious complications, it should be kept in mind that we should be always careful about possible complications, such as exacerbation of hepatitis due to the excessive enhancement of immune responses.
Fig. 5.
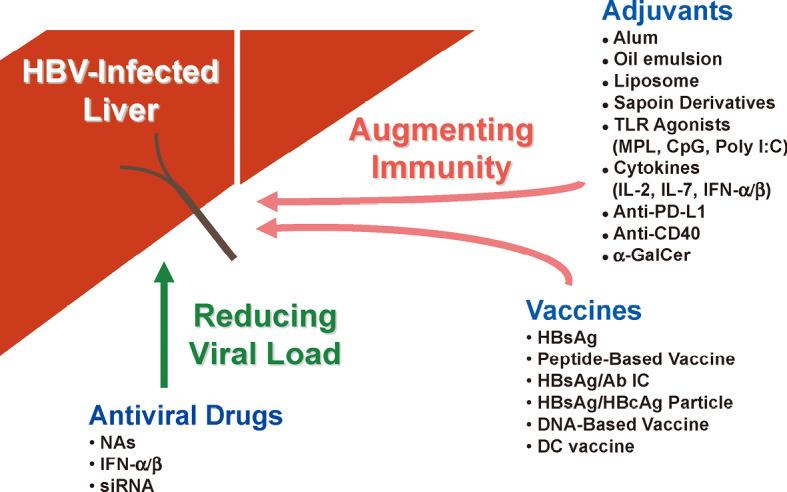
Summary of new strategies for treatment of chronic HBV infection. Strategies for CHB immunotherapy consist of novel vaccines, adjuvants, and combination of these to antiviral drugs. Details are described in the text.
REFERENCES
- 1).Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol, 1995; 13: 29–60. [DOI] [PubMed]
- 2).Ganem D, Prince AM. Hepatitis B virus infection-natural history and clinical consequences. N Engl J Med, 2004; 350: 1118–1129. [DOI] [PubMed]
- 3).Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol Mech Dis, 2006; 1: 23–61. [DOI] [PubMed]
- 4).Chen CJ, Yang HI, Su J, Jen CI, You SL, Lu SN, Huang GT, Iloeje UH. The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA, 2006; 295: 65–73. [DOI] [PubMed]
- 5).de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N. EASL International Consensus Conference on Hepatitis B on Hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement. J Hepatol, 2003; 39 Suppl 1: S3–S25. [PubMed]
- 6).Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman. Global cancer statistics. CA Cancer J Clin, 2011, 61: 69–90. [DOI] [PubMed]
- 7).World Health Organization. Vaccine-preventable diseases: monitoring system 2009 global summary. http://www.who. int/ immunization_monitoring/routine/ immunization_coverage/en/index4.html
- 8).Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination–worldwide, 2006. MMWR Morb Mortal Wkly Rep, 2008; 57: 1249–1252. [PubMed]
- 9).Ming L, Thorgeirsson SS, Gail MH, Lu P, Harris CC, Wang N, Shao Y, Wu Z, Liu G, Wang X, Sun Z. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology, 2002; 36: 1214–1220. [DOI] [PubMed]
- 10).Tanaka J, Kumagai J, Katayama K, Komiya Y, Mizui M, Yamanaka R, Suzuki K, Miyakawa Y, Yoshizawa H. Sex- and age-specific carriers of hepatitis B and C viruses in Japan estimated by the prevalence in the 3,485,648 first-time blood donors during 1995–2000. Intervirology, 2004; 47: 32–40. [DOI] [PubMed]
- 11).Uemura T, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. Hepatol Res, 2007; 37 (Suppl. 2): S95–S100. [DOI] [PubMed]
- 12).Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, the REVEAL-HBV Study Group. Predicting cirrhosis risk based on the levels of circulating hepatitis B viral load. Gastroenterology, 2006; 130: 678–686. [DOI] [PubMed]
- 13).Dienstag JL, Goldin RD, Heathcote EJ, Hann HWL, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology, 2003; 124: 105–117. [DOI] [PubMed]
- 14).Liaw YF, Sung JJY, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J, the Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med, 2004; 351: 1521–1531. [DOI] [PubMed]
- 15).Reijnders JGP, Janssen HLA. New approaches in the management of chronic hepatitis B: role of tenofovir. Infection Drug Resist, 2009; 2: 13–26. [DOI] [PMC free article] [PubMed]
- 16).Allen MI, Deslauriers M, Andrews CW, Tipples GA, Walters KA, Tyrrell DL, Brown N, Condreay LD. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology, 1998; 27: 1670–1677. [DOI] [PubMed]
- 17).Angus PR, Vaughan S, Xiong H, Yang H, Delaney W, Gibbs C, Brosgart C, Colledge D, Edwards R, Ayres A, Bartholomeusz A, Locarnini S. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology, 2003; 125: 292–297. [DOI] [PubMed]
- 18).Baldick CJ, Tenney DJ, Mazzucco CE, Eggers BJ, Rose RE, Pokornowski KA, Yu CF, Colonno RJ. Comprehensive evaluation of hepatitis B virus reverse transcriptase substitutions associated with entecavir resistance. Hepatology, 2008; 47: 1473–1482. [DOI] [PubMed]
- 19).Peters M. Actions of cytokines on the immune response and viral interactions: an overview. Hepatology, 1996; 23: 909–916. [DOI] [PubMed]
- 20).Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev, 2001; 14: 778–809. [DOI] [PMC free article] [PubMed]
- 21).van Nune AB, Hansen BE, Suh DJ, Löhr HF, Chemello L, Fontaine H, Heathcote J, Song BC, Janssen HLA, de Man RA, Schalm SW. Durability of HBeAg seroconversion following antiviral therapy for chronic hepatitis B: relation to type of therapy and pretreatment serum hepatitis B virus DNA and alanine aminotransferase. Gut, 2003; 52: 420–424. [DOI] [PMC free article] [PubMed]
- 22).Marcellin P, Lau GKK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S, Lai MY, Button P, Pluck N, the Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med, 2004; 351: 1206–1217. [DOI] [PubMed]
- 23).Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med, 2005; 352: 2682–2695. [DOI] [PubMed]
- 24).Janssen HLA, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TMK, Gerken G, de Man RA, Niesters HGM, Zondervan P, Hansen B, Schalm SW, the HBV 99-01 Study Group. Pegylated interferon alfa-2a alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomized trial. Lancet, 2005; 365: 123–129. [DOI] [PubMed]
- 25).Cooksley G, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, Chutaputti A, Chang WY, Zahm FE, Pluck N. Peginterferon alfa 2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepatitis, 2003; 10: 298–305. [DOI] [PubMed]
- 26).Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol, 2009; 50: 805–816. [DOI] [PubMed]
- 27).Couillin I, Pol S, Mancini M, Driss F, Bréchot C, Tiollais P, Michel ML. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J Infect Dis, 1999; 180; 15–26. [DOI] [PubMed]
- 28).Michel ML, Deng Q, Mancini-Bourgine M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: Perspectives and challenges. J Hepatol, 2011; 54: 1286–1296. [DOI] [PubMed]
- 29).Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol, 1999; 17:189–220. [DOI] [PubMed]
- 30).Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol, 2008; 8: 259–268. [DOI] [PMC free article] [PubMed]
- 31).Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell, 2004; 3: 177–184. [DOI] [PubMed]
- 32).Hansen DS, Schofield L. Regulation of immunity and pathogenesis in infectious diseases by CD1d-restricted NKT cells. Int J Parasitol, 2004; 34: 15–25. [DOI] [PubMed]
- 33).Subleski JJ, Wiltrout RH, Weiss JM. Application of tissue-specific NK and NKT cell activity for tumor immunotherapy. J Autoimmun, 2009; 33: 275–281. [DOI] [PMC free article] [PubMed]
- 34).Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: fact and controversies. Eur J Clin Invest, 2010; 40: 851–863. [DOI] [PubMed]
- 35).Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, Brown D, Gilson RJ, Tedder RJ, Dusheiko GM, Jacobs M, Klenerman P, Maini MK. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology, 2009; 137: 1289–1300. [DOI] [PubMed]
- 36).Timme R, Wieland S, Streiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol, 2003; 77: 68–76. [DOI] [PMC free article] [PubMed]
- 37).Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. Mechanisms of class I restricted immunopathology. A tramgenic mouse model of fulminant hepatitis. J Exp Med, 1993; 178: 1541–1554. [DOI] [PMC free article] [PubMed]
- 38).Ando K, Guidotti LG, WirthS, Ishikawa T, Missale G, Moriyama T, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J Immunol, 1994; 152: 3245–3253. [PubMed]
- 39).Ando K, Guidotti LG, Cerny A, Ishikawa T, Chisari FV. CTL access to tissue antigen is restricted in vivo. J Immunol, 1994; 153: 482–488. [PubMed]
- 40).Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA, 1994; 91: 3764–3768. [DOI] [PMC free article] [PubMed]
- 41).Guidotti LG, Hobbs MV, Ishikawa T, Matzke B, Schreiber RD, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity, 1996; 4: 25–36. [DOI] [PubMed]
- 42).Nakamoto Y, Guidotti LG, Pasquetto V, Schreiber RD, Chisari FV. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J Immunol, 1997; 158: 5692–5697. [PubMed]
- 43).Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol, 2001; 19: 65–91. [DOI] [PubMed]
- 44).Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte–induced liver damage. Nat Med, 2005; 11: 1167–1169. [DOI] [PMC free article] [PubMed]
- 45).Kakimi K, Lane TE, Wieland S, Asensio VC, Campbell IL, Chisari FV, Guidotti LG. Blocking chemokine responsive to γ-2/interferon (IFN)-γ inducible protein and monokine induced by IFN-γ activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J Exp Med, 2001; 194:1755–1766. [DOI] [PMC free article] [PubMed]
- 46).Sitia G, Isogawa M, Iannacone M, Campbell IL, Chisari FV, Guidotti LG. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J Clin Invest, 2004; 113:1158–1167. [DOI] [PMC free article] [PubMed]
- 47).McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol, 2000; 74: 2255–2264. [DOI] [PMC free article] [PubMed]
- 48).Wieland SF, Guidotti LG, Chisari FV. Intrahepatic induction of α/β interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol, 2000; 74: 4165–4173. [DOI] [PMC free article] [PubMed]
- 49).Robek MD, Wieland SF, Chisari FV. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J Virol, 2002; 76: 3570–3574. [DOI] [PMC free article] [PubMed]
- 50).Robek MD, Boyd BS,Wieland SF, Chisari FV. Signal transduction pathways that inhibit hepatitis B virus replication. Proc Natl Acad Sci USA, 2004; 101: 1743–1747. [DOI] [PMC free article] [PubMed]
- 51).Tsui LV, Guidotti LG, Ishikawa T, Chisari FV. Posttranscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc Natl Acad Sci USA, 1995; 92: 12398–12402. [DOI] [PMC free article] [PubMed]
- 52).Heise T, Guidotti LG, Chisari FV. La autoantigen specifically recognizes a predicted stem-loop in hepatitis B virus RNA. J Virol, 1999; 73: 5767–5776. [DOI] [PMC free article] [PubMed]
- 53).Heise T, Guidotti LG, Chisari FV. Characterization of nuclear RNases that cleave hepatitis B virus RNA near the La protein binding site. J Virol, 2001; 75: 6874–6883. [DOI] [PMC free article] [PubMed]
- 54).Heise T, Guidotti LG, Cavanaugh VJ, Chisari FV. Hepatitis B virus RNA-binding proteins associated with cytokine-induced clearance of viral RNA from the liver of transgenic mice. J Virol, 1999; 73: 474–481. [DOI] [PMC free article] [PubMed]
- 55).Horke S, Reumann K, Rang A, Heise T. Molecular characterization of the human La protein•hepatitis B virus RNA.B interaction in vitro. J Biol Chem, 2002; 277: 34949–34958. [DOI] [PubMed]
- 56).Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science, 1999; 284: 825–829. [DOI] [PubMed]
- 57).Guidotti LG, McClary H, Loudis JM, Chisari FV. Nitric oxide inhibits hepatitis B virus replication in the livers of transgenic mice. J Exp Med, 2000; 191: 1247–1252. [DOI] [PMC free article] [PubMed]
- 58).Franco A, Guidotti LG, Hobbs MV, Pasquetto V, Chisari FV. Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J Immunol, 1997; 159: 2001–2008. [PubMed]
- 59).Bertoletti A, Tan AT, Gehring AJ. HBV-specific adaptive immunity. Viruses, 2009; 1: 91–103. [DOI] [PMC free article] [PubMed]
- 60).Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest, 2004; 114: 701–712. [DOI] [PMC free article] [PubMed]
- 61).Clispe NI. Hepatic T cells and liver tolerance. Nat Rev Immunol, 2003; 3: 51–62. [DOI] [PubMed]
- 62).Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol, 2005; 26: 512–517. [DOI] [PubMed]
- 63).Akbar SMF, Horiike N, Onji M, Hino O. Dendritic cells and chronic hepatitis virus carriers. Intervirology, 2001; 44: 199–208. [DOI] [PubMed]
- 64).Woltman AM, Boonstra A, Janssen HL. Dendritic cells in chronic viral hepatitis B and C: victims or guardian angels? Gut, 2010; 59: 115–125. [DOI] [PubMed]
- 65).van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J, Janssen HLA. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology, 2004; 40: 738–746. [DOI] [PubMed]
- 66).Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol, 2007; 19: 813–824. [DOI] [PubMed]
- 67).Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA, 2004; 101: 16004–16009. [DOI] [PMC free article] [PubMed]
- 68).Wherry EJ. T cell exhaustion. Nat Immunol, 2011; 12: 492–499. [DOI] [PubMed]
- 69).Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G, Ferrari C. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology, 2010; 138: 682–693. [DOI] [PubMed]
- 70).Stoop JN, van der Molen RG, Baan CC, van der Laan LJW, Kuipers EJ, Kusters JG, Janssen HLA. Regulatory T Cells Contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology, 2005; 41: 771–778. [DOI] [PubMed]
- 71).Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, Tang Z, Fu YX, Wang FS. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol, 2006; 177: 739–747. [DOI] [PubMed]
- 72).Stoop JN, Claassen MA, Woltman AM, Binda RS, Kuipers EJ, Janssen HLA, van der Molen RG, Boonstra A. Intrahepatic regulatory T cells are phenotypically distinct from their peripheral counterparts in chronic HBV patients. Clin Immunol, 2008; 129: 419–427. [DOI] [PubMed]
- 73).Franzese O, Kennedy PT, Gehring AJ, Gotto J, Williams R, Maini MK, Bertoletti A. Modulation of the CD8+-T-cell response by CD4+CD25+ regulatory T cells in patients with hepatitis B virus infection. J Virol, 2005; 79: 3322–3328. [DOI] [PMC free article] [PubMed]
- 74).Pol S, Driss F, Carnot F, et al. Vaccination against hepatitis B virus: an efficient immunotherapy against hepatitis B multiplication. C R Acad Sci III, 1993; 316: 688–691. [PubMed]
- 75).Pol S, Driss F, Michel ML, Nalpas B, Berthelot P, Bréchot C. Specific vaccine therapy in chronic hepatitis B infection. Lancet, 1994; 344: 342. [DOI] [PubMed]
- 76).Pol S, Nalpas B, Driss F, Michel ML, Tiollais P, Denis J, Bréchot C, Multicenter study group. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J Hepatol, 2001; 34: 917–921. [DOI] [PubMed]
- 77).Jung MC, Gruner N, Zachoval R, Schraut W, Gerlach T, Diepolder H, Schirrena CA, Page M, Bailey J, Birtles E, Whitehead E, Trojan J, Zeuzem S, Pape GR. Immunological monitoring during therapeutic vaccination as a prerequisite for the design of new effective therapies: induction of a vaccine-specific CD4+ T-cell proliferative response in chronic hepatitis B carriers. Vaccine, 2002; 20: 3598–3612. [DOI] [PubMed]
- 78).Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HWL, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M, Brown NA, the US Lamivudine Investigator Group. Lamvudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med, 1999; 341: 1256–1263. [DOI] [PubMed]
- 79).Lai CL, Chein RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J, Stephenson SL, Gray DF, the Asia Hepatitis Lamivudine Study Group. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med, 1998; 339: 61–68. [DOI] [PubMed]
- 80).Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R, Fiaccadori F, Ferrari C. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest, 1998; 102: 968–975. [DOI] [PMC free article] [PubMed]
- 81).Boni C, Penna A, Bertoletti A, Lamonaca V, Rapti I, Missale G, Pilli M, Urbani S, Cavalli A, Cerioni S, Panebianco R, Jenkins J, Ferrari C. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J Hepatol, 2003; 39: 595–605. [DOI] [PubMed]
- 82).Horiike N, Akbar SMF, Michitaka K, Joukou K, Yamamoto K, Kojima N, Hiasa Y, Abe M, Onji M. In vivo immunization by vaccine therapy following virus suppression by lamivudine: a novel approach for treating patients with chronic hepatitis B. J Clin Virol, 2005; 32: 156–161. [DOI] [PubMed]
- 83).Vandepapeliere P, Lau GK, Leroux-Roels G, Horsmans Y, Gane E, Tawandee T, bin Merican MI, Wing KM, Trepo C, Cooksley G, Wettendorff M, Ferrari C, The Therapeutic HBV Vaccine Group of Investigators. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of coadministration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine, 2007; 25: 8585–8597. [DOI] [PubMed]
- 84).Senturk H, Tabak F, Ozaras R, Erdem L, Canbakan B, Mert A, Yurdakul I. Efficacy of pre-S-containing HBV vaccine combined with lamivudine in the treatment of chronic HBV infection. Dig Dis Sci, 2009; 54: 2026–2030. [DOI] [PubMed]
- 85).Ishikawa T, Kakumu S. Combination therapy with lamivudine and HB vaccine on chronic hepatitis B. Hepatol Res, 2007; 37: S62–S66. [DOI] [PubMed]
- 86).Miquilena-Colina ME, Lozano-Rodríguez T, García-Pozo, Sáeza A, Rizza P, Capone I, Rapicetta M, Chionne P, Capobianchi M, Selleri M, Castilletti C, Belardelli F, Iacono OL, García-Monzón C. Recombinant interferon-α2b improves immune response to hepatitis B vaccination in haemodialysis patients: Results of a randomised clinical trial. Vaccine, 2009; 27: 5654–5660. [DOI] [PubMed]
- 87).Heintges T, Petry W, Kaldewey M, Erhardt A, Wend UC, Gerlich WH, Niederau C, Hãussinger D. Combination therapy of active HBsAg vaccination and interferon-α in interferon-α nonresponders with chronic hepatitis B. Dig Dis Sci, 2001; 46: 901–906. [DOI] [PubMed]
- 88).Helvaci M, Kizilgunesler A, Kasirga E, Ozbal E, Kuzu M, Sozen G. Efficacy of hepatitis B vaccination and interferon-α-2b combination therapy versus interferon-α-2b monotherapy in children with chronic hepatitis B. J Gastroenterol Hepatol, 2004; 19: 785–791. [DOI] [PubMed]
- 89).Han Q, Zhang C, Zhang J, Tian Z. Reversal of hepatitis B virus-induced immune tolerance by an immunostimulatory 3p-HBx-siRNAs in a retinoic acid inducible gene I–dependent manner. Hepatology, 2011; 54: 1179–1189. [DOI] [PubMed]
- 90).Cox JC, Coulter AR. Adjuvants -a classification and review of their modes of action. Vaccine, 199; 15: 248–256. [DOI] [PubMed]
- 91).Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol, 2008; 30: 23–32. [DOI] [PubMed]
- 92).Sablan BP, Kim DJ, Barzaga NG, Chow WC, Cho M, Ahn SH, Hwang SG, Lee JH, Namini H, Heyward WL. Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine, 2012; 30: 2689–2696. [DOI] [PubMed]
- 93).Dahmen A, Herzog-Hauff S, Böcher WO, Galle PR, Löhr HF. Clinical and immunological efficacy of intradermal vaccine plus lamivudine with or without interleukin-2 in patients with chronic hepatitis B. J Med Virol, 2002; 66: 452–460. [DOI] [PubMed]
- 94).Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, Assouline B, Lahl K, Sparwasser T, Ohashi PS, Mak TW. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med, 2009; 15: 528–536. [DOI] [PubMed]
- 95).Watanabe T, Bertoletti A, Tanoto TA. PD-1/PD-L1 pathway and T-cell exhaustion in chronic hepatitis virus infection. J Viral Hepat, 2010; 17: 453–458. [DOI] [PubMed]
- 96).Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J Immunol, 2002; 169: 5188–5195. [DOI] [PubMed]
- 97).Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol, 2004; 22: 307–328. [DOI] [PubMed]
- 98).Ito H, Ando K, Ishikawa T, Nakayama T, Taniguchi M, Saito K, Imawari M, Moriwaki H, Yokochi T, Kakumu S, Seishima M. Role of Vα14+ NKT cells in the development of hepatitis B virus-specific CTL: activation of Vα14+ NKT cells promotes the breakage of CTL tolerance. Int Immunol, 2008; 20: 869–879. [DOI] [PubMed]
- 99).Livingston BD, Crimi C, Grey H, Ishioka G, Chisari FV, Fikes J, Grey H, Chesnut RW, Sette A. The hepatitis B virus-specific CTL responses induced in humans by lipopeptide vaccination are comparable to those elicited by acute viral infection. J Immunol, 1997; 159: 1383–1392. [PubMed]
- 100).Livingston BD, Alexander J, Crimi C, Oseroff C, Celis E, Daly K, Guidotti LG, Chisari FV, Fikes J, Chesnut RW, Sette A. Altered helper T lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in humans. J Immunol, 1999; 162: 3088–3095. [PubMed]
- 101).Ishikawa T, Kono D, Chung J, Fowler P, Theofilopoulos A, Kakumu S, Chisari FV. Polyclonality and multispecificity of the CTL response to a single viral epitope. J Immunol, 1998; 161: 5842–5850. [PubMed]
- 102).Xu DZ, Zhao K, Guo LM, Chen XY, Wang HF, Zhang JM, Xie Q, Ren H, Wang WX, Li LJ, Xu M, Liu P, Niu JQ, Bai XF, Shen XL, Yuan ZH, Wang XY, Wen YM. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS ONE, 2008; 3: e2565. [DOI] [PMC free article] [PubMed]
- 103).Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection. Int J Mol Sci, 2010; 11: 789–806. [DOI] [PMC free article] [PubMed]
- 104).Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med, 1995; 181: 1047–1058. [DOI] [PMC free article] [PubMed]
- 105).Aguilar JC, Lobaina Y, Muzio V, García D, Pentón E, Iglesias E, Pichardo D, Urquiza D, Rodríguez D, Silva D, Petrovsky N, Guillén G. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol Cell Biol, 2004; 82: 539–546. [DOI] [PubMed]
- 106).Betancourt AA, Delgado CA, Estéveza ZC, Martínez JC, Ríos GV, Aureoles-Rosellóc SRM, Zaldívara RA, Guzmána MA, N. Baile F, Reyes PAD, Ruano LO, Fernández AC, Lobaina-Matos Y, Fernández AD, Madrazo AIJ, Martínez MIA, Baños ML, Alvarez NP, Baldo MD, Mestre RES, Pérez MVP, Martínez MEP, Escobar DA, Guanche MJC, Cáceres LM, Betancourt RS, Rando EH, Nieto, GEG, González VLM, Rubido JCA. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int J Infect Dis, 2007; 11: 394–401. [DOI] [PubMed]
- 107).Mancini-Bourgine M, Fontaine H, Scott-Algara D, Pol S, Bréchot C, Michel ML. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology, 2004; 40: 874–882. [DOI] [PubMed]
- 108).Scott-Algara D, Mancini-Bourgine M, Fontaine H, Pol S, Michel ML. Changes to the natural killer cell repertoire after therapeutic hepatitis B DNA vaccination. PLoS ONE, 2010; 5: e8761. [DOI] [PMC free article] [PubMed]
- 109).Akbar SMF, Furukawa S, Horiike N, Abe M, Hiasa Y, Onji M. Safety and immunogenicity of hepatitis B surface antigen-pulsed dendritic cells in patients with chronic hepatitis B. J Viral Hepat, 2011; 18: 408–414. [DOI] [PubMed]
- 110).Luo J, Li J, Chen RL, Nie L, Huang J, Liu ZW, Luo L, Yan XJ. Autologus dendritic cell vaccine for chronic hepatitis B carriers: a pilot, open label, clinical trial in human volunteers. Vaccine, 2010; 28: 2497–2504. [DOI] [PubMed]