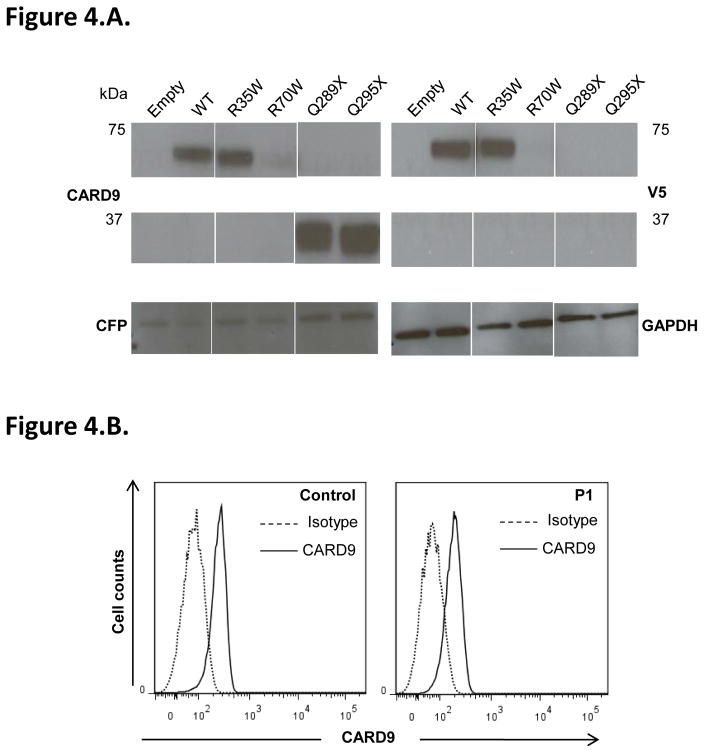
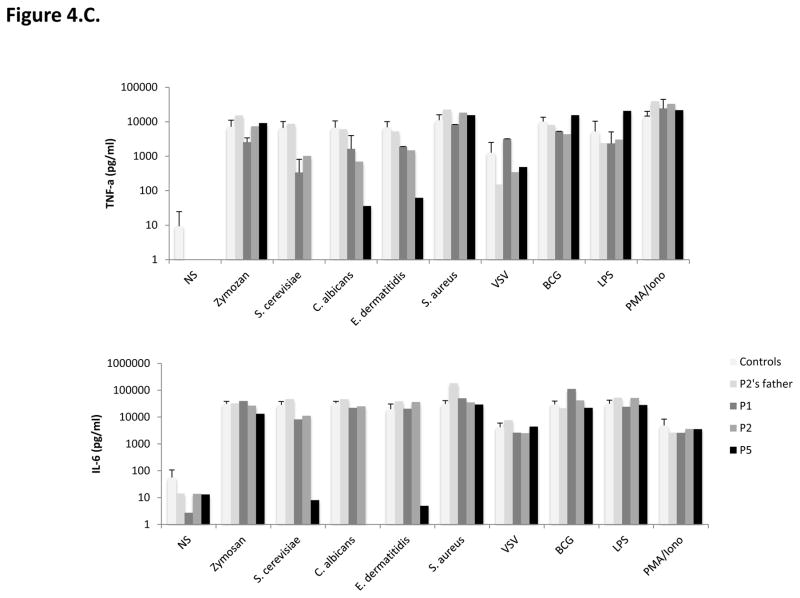
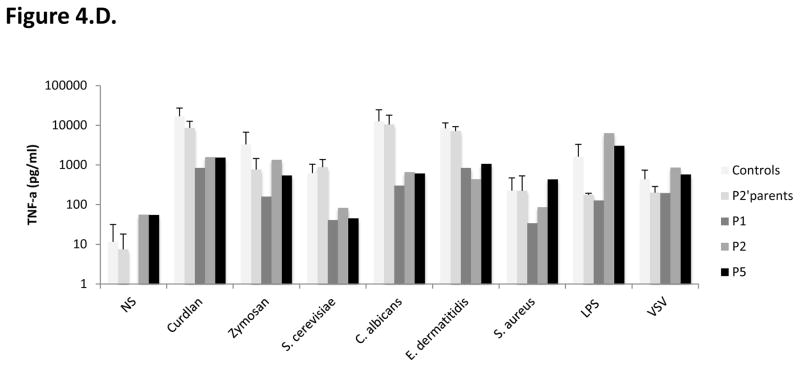
Figure 4. Impact of CARD9 mutations on CARD9 protein levels and function.
A–B. Impact of CARD9 mutations on CARD9 protein levels. A. Immunoblot analysis of CARD9 in whole-cell extracts of HEK-293T cells co-transfected with pcDNA3.1 V5 (C-terminally tagged), either empty or carrying the wild-type (WT) or mutant (R35Q, R70W, Q289* and Q295*) CARD9 alleles, together with a CFP plasmid, as a transfection control. Antibodies against CARD9, V5, CFP and GAPDH (as a loading control) were used. B. Flow cytometry analysis of CARD9 expression in monocyte-derived dendritic cells (MDDCs) from patient P1 and a control.
C–D. Impact of CARD9 mutations on CARD9 protein function. C. TNF-α (top panel) and Interleukin (IL)-6 (bottom panel) production by whole blood cells after 24 hours of stimulation with zymosan, heat-killed S. cerevisiae, C. albicans, E. dermatitidis, S. aureus, VSV, BCG, LPS, and PMA plus ionomycin for P1, P2, P5, P2’s father and 7 controls. D. TNF-α production after 24 hours of stimulation of MDDCs with curdlan, zymosan, S. cerevisiae, C. albicans, E. dermatitidis, S. aureus, LPS and VSV for P1, P2 and P5, P2’s parents, and 6 healthy controls tested in parallel.