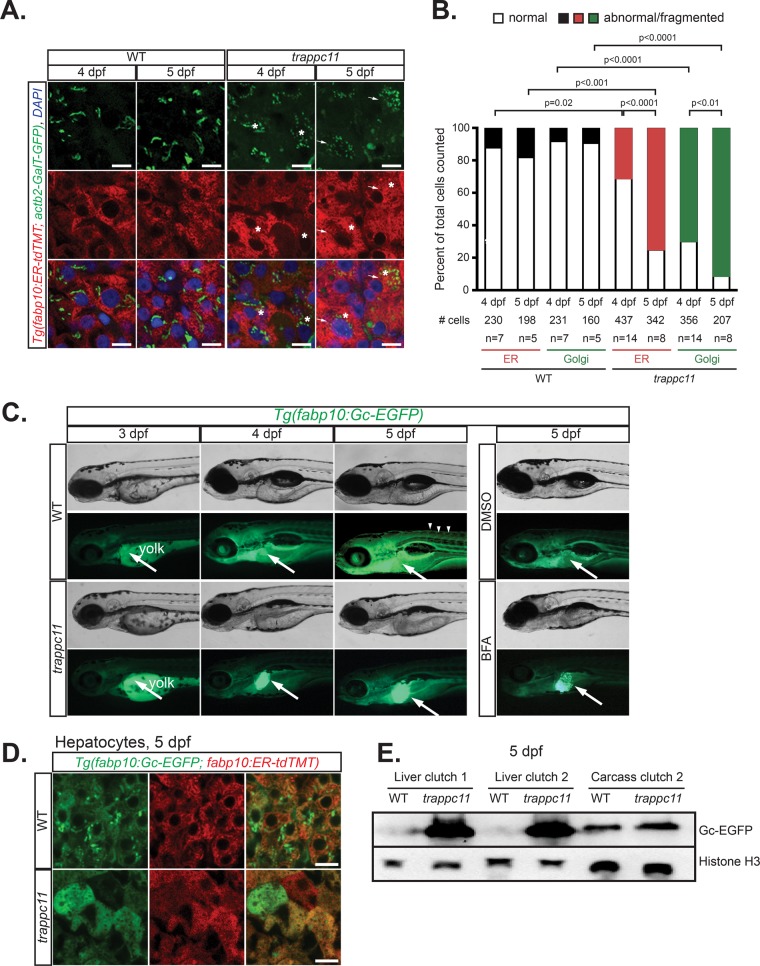
FIGURE 2:
Mutation of trappc11 in zebrafish causes secretory pathway defects. (A) Confocal microscopy on cryosections from 4- and 5-dpf trappc11 mutants and phenotypically WT siblings that ubiquitously express a Golgi apparatus marker (green), Tg(actb2:GalT-GFP), and a hepatocyte-specific ER marker (red), Tg(fabp10:ER-td-TMT). Hepatocytes that show abnormal morphology, that is, fragmented Golgi apparatus and distended ER, are marked with arrows and asterisks, respectively, and quantified (B) as percentage abnormal to total hepatocytes counted (# cells) from number of fish counted (n). The p values were calculated using Fisher’s exact test. (C) Bright-field and fluorescence images of 3- to 5-dpf WT and trappc11 transgenic Tg(fabp10:Gc-EGFP) larvae demonstrate GFP accumulation in the liver of trappc11 mutants, similar to that seen with BFA-treated (1 μg/ml) control larvae, which indicates defective protein secretion. Arrows indicate livers where Gc-EGFP is being produced, and arrowheads show secreted Gc-EGFP in the vasculature. (D) Confocal images of livers from 5-dpf WT and trappc11 mutants that express both the secreted Gc-EGFP (green) protein and the ER marker ER-tdTMT (red) confirms accumulation in the ER of mutant hepatocytes, evidenced by colocalization of both markers. (E) Western analysis of WT and trappc11-mutant livers using anti-GFP to detect Gc-EGFP and anti-Histone H3 as a loading control. Two independent clutches are shown for liver samples. Eight pooled livers and one liverless carcass were loaded. Bars, 10 μm (A, D).