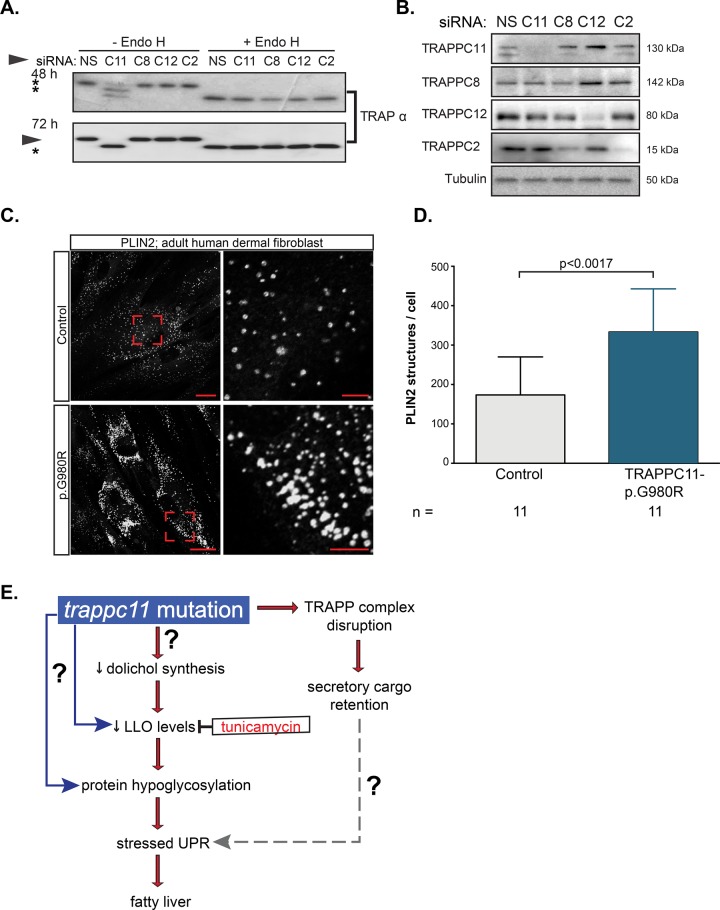
FIGURE 7:
TRAPPC11 in human cells is required for glycosylation and for inhibiting lipid accumulation. (A) HeLa cells were transfected with either a nonspecific siRNA (NS) or siRNA targeting TRAPPC11 (C11), TRAPPC8 (C8), TRAPPC12 (C12), or TRAPPC2 (C2) transcripts and probed after 48 and 72 h for glycosylation of the ER-resident protein TRAPα. Asterisks indicate hypoglycosylated (unglycosylated and monoglycosylated) moieties of TRAPα. Representative Western blot of three experiments. Endo H digestion was performed to indicate fully hypoglycosylated high-mannose-containing species. (B) siRNA-mediated knockdown of TRAPP components shown in A were confirmed by Western blot using antibodies against TRAPPC11, TRAPPC8, TRAPPC12, and TRAPPC2. Tubulin was used as a loading control. Molecular size of TRAPP members is shown on the right. (C) Dermal fibroblasts from control or a patient harboring the homozygous missense mutation p.G980R in TRAPPC11 were fixed and stained with anti-PLIN2 antibody, followed by Alexa Fluor 647. Scale bars, 25 μm (left), 5 μm (right). The images are representative of three independent experiments. (D) Quantification of the PLIN2-positive structures per cell confirms a significant increase in PLIN2-positive structures in p.G980R patient cells compared with control cells (Student’s t test). Number of cells counted (n) is indicated. (E) Working model for mechanism by which trappc11 mutation causes a stressed UPR and fatty liver. Both protein glycosylation and protein trafficking are blocked in trappc11 mutants, and our data suggest that the protein glycosylation defect, either alone or with the added cellular stress caused by secretory cargo retention, leads to a stressed UPR, which causes fatty liver.