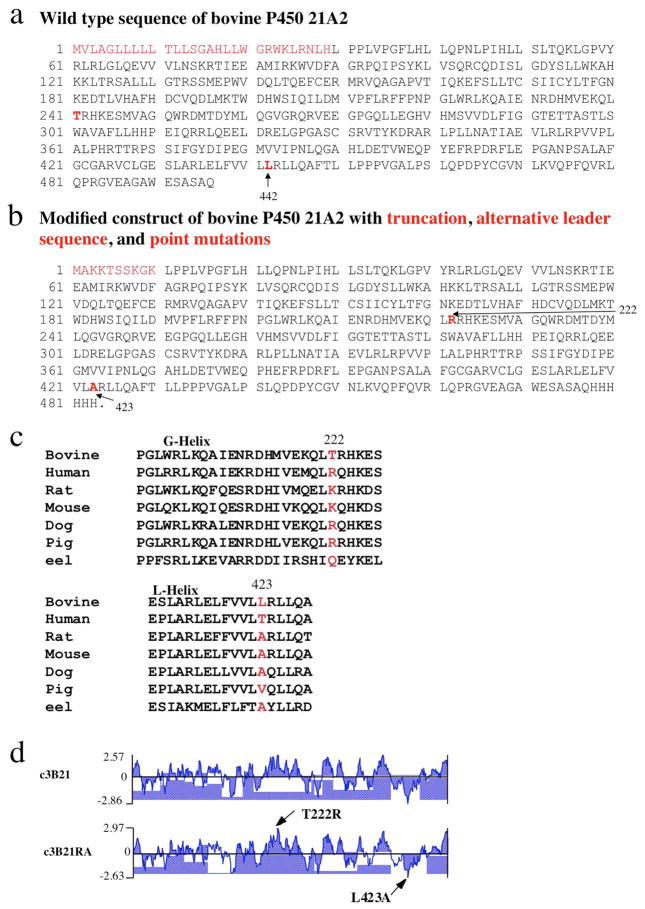
Figure 2.
Generation of alternative P450 constructs for improving expression, solubility, stability and crystal formation, using bovine P450 21A2 as an example (Zhao et al., 2012). (a) Wild type sequence of bovine P450 21A2. (b) A soluble fragment was obtained by replacing the hydrophobic N-terminal 29 amino acids in bovine P450 21A2 by the shorter MAKKTSSKGK leader sequence (from the N-terminus of P450 2C3). Residues that were mutated individually are highlighted in red in the wild type (Thr-241 and Leu-442) and alternative protein constructs (Arg-222 and Ala-423, respectively). (c) Identification of candidate surface residues for point mutation (T241R and L442A) by aligning the sequences of P450 21A2 from selected eukaryotic species, combined with (d) hydrophilicity analyses of wild type (with alternative leader; c3B21) and mutated protein sequences (c3B21RA). The alternative protein construct shown in panel b was used for determining the crystal structure of bovine P450 21A2 (Zhao et al., 2012).