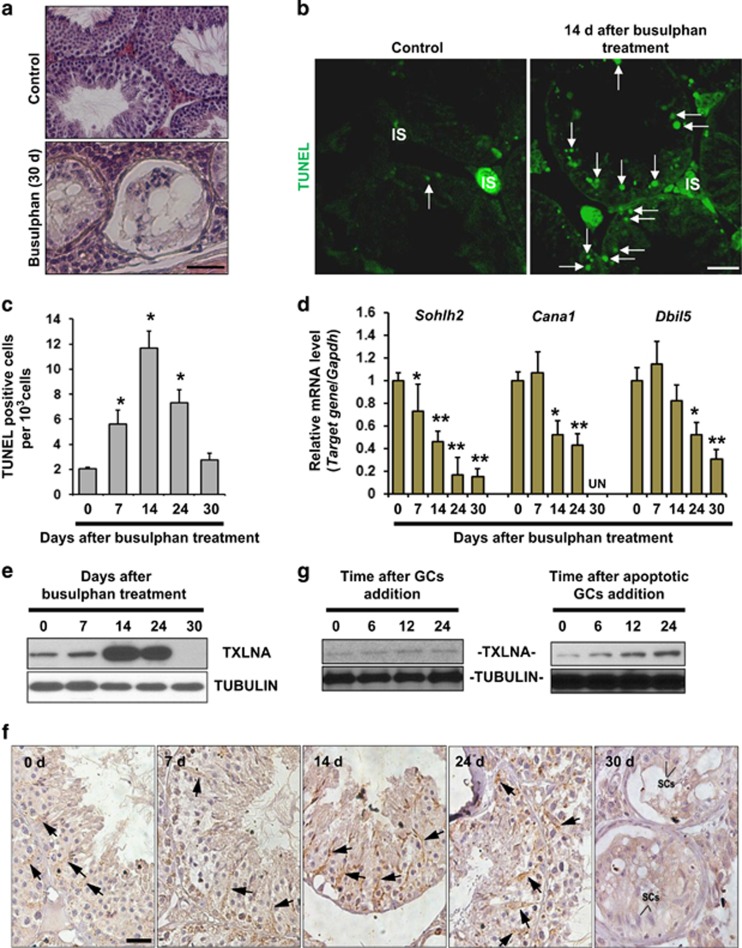
Figure 2.
Presence of TXLNA in SCs is required for proper contact between SCs and adjacent germ cells (GCs). (a) Tissue sections show morphology in control testes and busulfan-treated testes 30 d after treatment. At this time point, seminiferous tubules were largely devoid of GCs. Scale bar, 25 μm. (b) TUNEL staining of testicular sections was carried out at 14 d after busulfan treatment. Nuclear green signal indicates apoptotic cells (white arrows). IS, testicular interstitium. Scale bar, 25 μm. (c) Quantification of TUNEL-positive cells. Results are expressed as mean±S.E.M.; n=3 independent experiments (*P<0.05, when compared to the value at 0 d, Student's t-test). (d) The deleterious effects of busulfan treatment on the spermatogenic differentiation at different time points was evaluated by qRT-PCR analyses using specific primers for mouse Sohlh2 (differentiating spermatogonia), for mouse Ccna1 (pachytene spermatocytes) and for mouse Dbil5 (haploid spermatids), respectively. Gapdh served as an internal control. Results presented as mean±S.E.M. of three independent experiments. (*P<0.05 or **P<0.01, when compared to the value at 0 d, Student's t-test). (e) Effect of busulfan treatment on testicular TXLNA expression was monitored using immunoblotting analyses. Tubulin served as a loading control. (f) Localization of testicular TXLNA protein at different time points after busulfan treatment was evaluated using immunohistochemistry. Scale bar, 25 μm. (g) Western blotting analysis of TXLNA protein in cultured SCs at different time points after incubation with freshly isolated GCs or with apoptotic GCs. Tubulin was used as a loading control