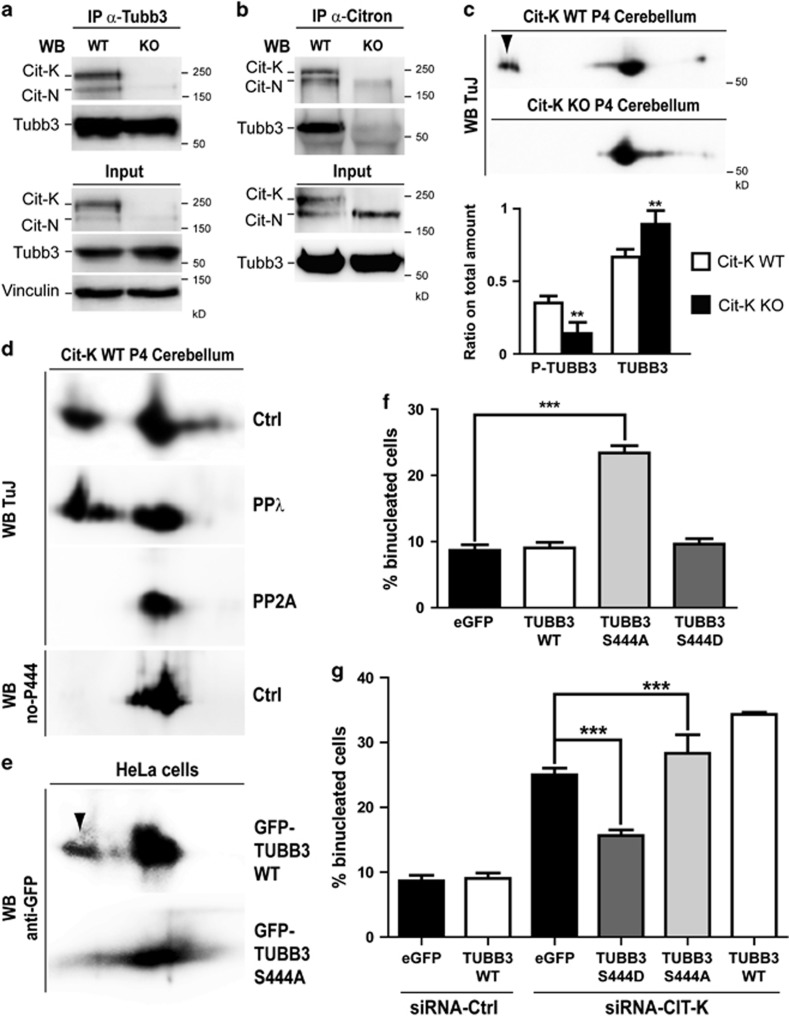
Figure 5.
CIT-K physically associates with Tubb3 in proliferating CNS and affects the phosphorylation of its S444 residue. (a and b) Total cell lysates from P4 wild-type (WT) and Cit-K knockout (KO) mouse cerebellum were immunoprecipitated (IP) with antibodies recognizing Tubb3 (a) or Citron proteins (b). Immunoprecipitates and total lysates (Input) were then fractionated by SDS-PAGE and analyzed for western blotting (WB) with the indicated antibodies. (c) Total cell lysates from P4 WT and Cit-K KO mouse cerebellum were separated by 2D gel electrophoresis and analyzed by WB using the anti-Tubb3 TuJ antibody. The arrowhead indicates an acidic spot detected in the WT but not in the KO samples. The relative amount of protein in the acidic spot (phosphorylated) or in the main spot (nonphosphorylated) was quantified in the lower panel. (d) Total cell lysates from P4 WT mouse cerebellum, untreated (Ctrl) or treated with phage protein phosphatase-λ (PPλ) or with erythrocyte protein phosphatase-2A (PP2A) were analyzed by 2D WB using the anti-Tubb3 antibodies TuJ or no-P444 (not recognizing the phosphorylated-S444 isoform). (e) Total cell lysates from HeLa cells transiently transfected with the indicated eGFP-TUBB3 fusion constructs were separated by 2D gel electrophoresis and analyzed by WB using the anti-GFP antibodies. The arrowhead indicates an acidic spot detected with the WT construct but not with the S444A mutant. (f and g) HeLa cells were transiently transfected with eGFP or with the indicated eGFP fusion constructs and after 24 h were transfected with control (CTRL) or with CIT-K-specific siRNAs. After 48 h, the percentage of binucleated cells was quantified as above. All the results are representative of at least three independent experiments (30 fields for each experiment). Error bars=S.E.M. Statistical significance of differences in average was established using Student's two-tailed t-test. **P<0.01; ***P<0.001