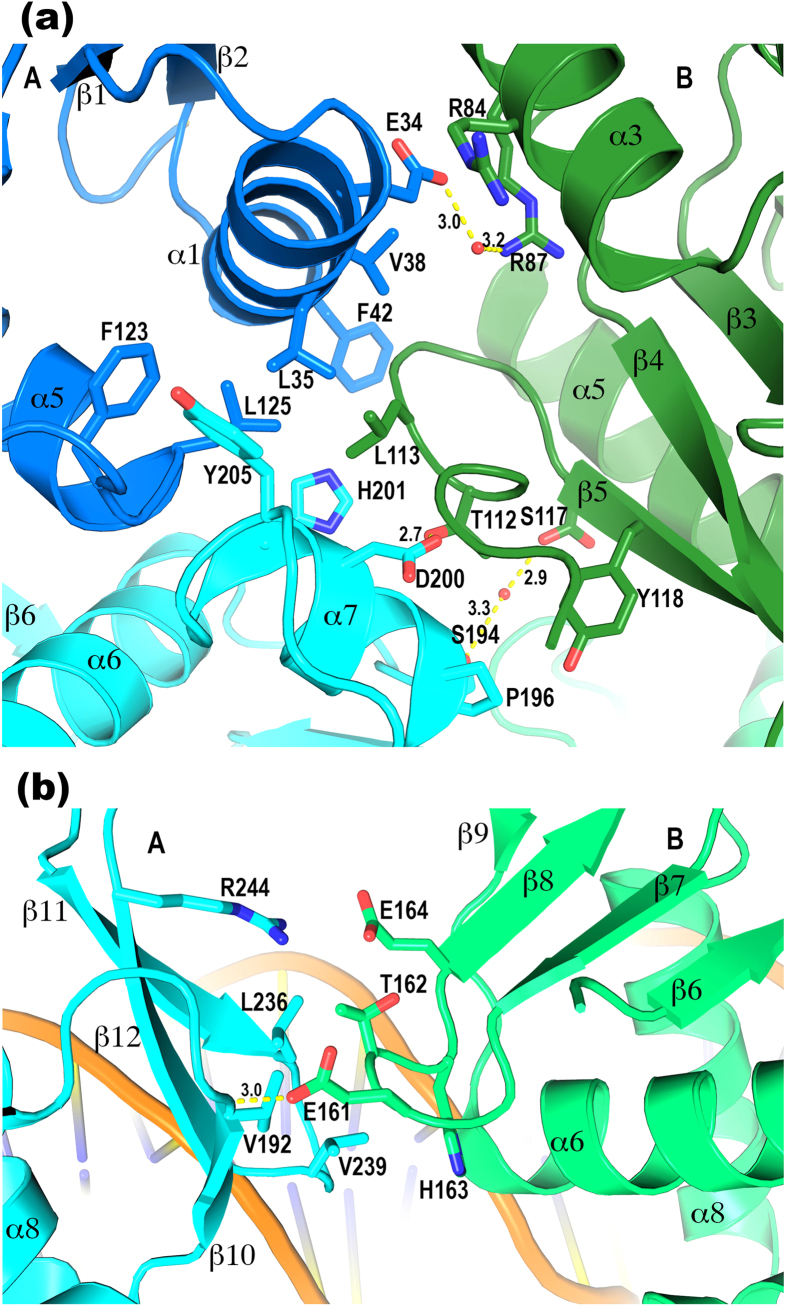
Figure 2. PhoP tandem dimer interface.
(a) The major patch of the dimer interface where both domains of subunit A interact with the RD of subunit B. The colour scheme is identical to that in Fig. 1. The side chains involved in the dimer interface are shown as sticks. Hydrogen bonds are shown as yellow dashed lines with the distances labelled in Å units. The Leu113 in B has hydrophobic interactions with the Leu35, Leu125, and His201 in A. The Tyr118 in B has hydrophobic interactions with the Pro196 in A. The Phe42 side chain stacks with the peptide plane of Gly114, and the Arg84 guanidinium group stacks with the peptide plane of Asn31 at the N-terminus of α1, both at a distance of ~4 Å. (b) The minor patch of the dimer interface where the DBDs of the two subunits meet. The RD of A is coloured in cyan and B is in light green. A hydrophobic patch in A consisting of the side chains Val192, Leu236, and Val239 interacts with the hydrophobic parts of the Glu161, Thr162, and His163 in B.