Figure 7.
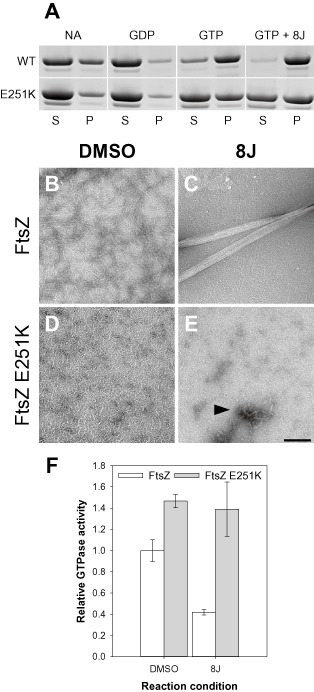
FtsZ E251K assembles in vitro in a benzamide‐independent manner.
A. Sedimentation assays of FtsZ and FtsZ E251K. Proteins (10 μM) were prepared in polymerisation buffer (50 mM HEPES pH 6.8; 50 mM KCl; 10 mM MgCl2) with no additions (NA), or in the presence of GDP (1 mM), GTP (1 mM) and GTP + 8J (1 mM and 20 μg ml−1), as indicated. Reactions were incubated at 30°C for 10 min, and assembly was assayed by sedimentation. S, supernatant; P, pellet.
B–E. Examination of FtsZ polymers by electron microscopy. FtsZ and FtsZ E251K (10 μM) were assembled in polymerisation buffer in the absence (B and D) and presence (C and E) of 8J (20 μg ml−1). Polymerisation was initiated by the addition of GTP (1 mM), and reactions were incubated at 30°C for 10 min before being prepared for electron microscopy. Scale bar = 200 nm.
F. FtsZ E251K GTPase activity is unaffected by 8J. FtsZ and FtsZ E251K (10 μM) were prepared in polymerisation buffer in the absence (DMSO only) and presence of 8J (20 μg ml−1). Polymerisation was initiated by the addition of GTP (1 mM), and the reactions were incubated at 30°C for 30 min. GTPase activity was determined using the malachite green assay, and for each condition, the mean of three independent experiments was plotted. Error bars represent one standard deviation.
