Figure 4.
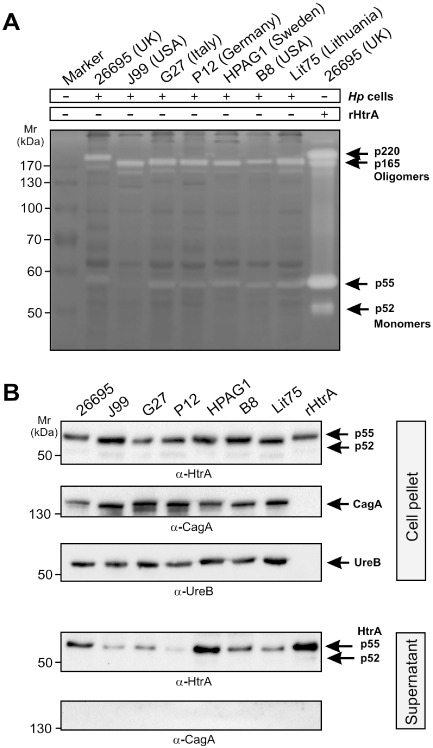
HtrA protein expression, oligomer formation and secretion in a large set of strains.
A. Casein zymography of the indicated H . pylori strains reveals proteolytically active HtrA protein species of 55 kDa, 165 kDa and 220 kDa as labelled with arrows. Purified recombinant HtrA of 26695 is loaded in lane 9 and produced the same pattern of active HtrA forms.
B. H . pylori isolates were grown in liquid BHI medium for 12 h and then fractionated in bacterial cell pellets (top) and supernatants (bottom). Immunoblotting using α‐HtrA antibodies show that all H . pylori strains exhibit strong HtrA signals in the supernatant fraction but varied substantially in their band intensities. As control, the bacterial pellets and supernatants were probed with α‐CagA and α‐UreB antibodies, excluding artificially lysed bacteria.
