Scheme 3.
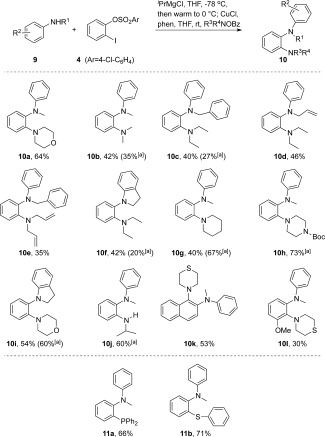
Synthesis of 2‐aminoaniline. Reaction conditions: amine (1 mmol), iPrMgCl (2.2 mmol), and aryne precursor (1.2 mmol) were stirred at −78 °C for 45 min, then warmed to 0 °C. The resulting intermediate Grignard was quenched with R1R2N—OBz (1.5 mol) in the presence of CuCl (10 mol %) and phenanthroline (10 mol %; see SI). [a] An additional zincation step was performed by adding ZnCl2 (0.5 equiv) after the initial anilide addition. Bz=benzoyl, Boc=tert‐butyloxycarbonyl.
