Abstract
Even with current promising antitumor antibodies, their antitumor effects on stroma‐rich solid cancers have been insufficient. We used mild hyperthermia with the intent of improving drug delivery by breaking the stromal barrier. Here, we provide preclinical evidence of cetuximab + mild hyperthermia therapy. We used four in vivo pancreatic cancer xenograft mouse models with different stroma amounts (scarce, MIAPaCa‐2; moderate, BxPC‐3; and abundant, Capan‐1 and Ope‐xeno). Cetuximab (1 mg/kg) was given systemically, and the mouse leg tumors were concurrently heated using a water bath method for 30 min at three different temperatures, 25°C (control), 37°C (intra‐abdominal organ level), or 41°C (mild hyperthermia) (n = 4, each group). The evaluated variables were the antitumor effects, represented by tumor volume, and in vivo cetuximab accumulation, indirectly quantified by the immunohistochemical fluorescence intensity value/cell using antibodies against human IgG Fc. At 25°C, the antitumor effects were sufficient, with a cetuximab accumulation value (florescence intensity/cell) of 1632, in the MIAPaCa‐2 model, moderate (1063) in the BxPC‐3 model, and negative in the Capan‐1 and Ope‐xeno models (760, 461). By applying 37°C or 41°C heat, antitumor effects were enhanced shown in decreased tumor volumes. These enhanced effects were accompanied by boosted cetuximab accumulation, which increased by 2.8‐fold (2980, 3015) in the BxPC‐3 model, 2.5‐ or 4.8‐fold (1881, 3615) in the Capan‐1 model, and 3.2‐ or 4.2‐fold (1469, 1922) in the Ope‐xeno model, respectively. Cetuximab was effective in treating even stroma‐rich and k‐ras mutant pancreatic cancer mouse models when the drug delivery was improved by combination with mild hyperthermia.
Keywords: Cetuximab, drug delivery, mild hyperthermia, monoclonal antibody, pancreatic cancer
Pancreatic cancer is still refractory to treatment, with a 5‐year survival rate of 5–7%.1, 2 This limited treatment efficacy may be attributable to histological features, such as hypovascularity,3 and the production of abundant interstitial stroma that surrounds the cancer cells, which is referred to as a desmoplastic reaction.4 As a result, few chemotherapeutic drugs enter the cancer nodule. Moreover, only a very small proportion of the drug actually reaches the cancer cells because the subsequent diffusion is hampered by the thick interstitial stroma.
Heat has been used to eradicate cancers since the time of Hippocrates (460–370 bc). A subcategory of hyperthermia, referred to as mild hyperthermia, has been in use since the 1970s. Mild hyperthermia involves using a temperature of 41–43°C to improve the effect of co‐administered anticancer drugs and radiation.5 The reasoning behind this approach is that the application of mild heat increases blood flow and the permeability of tumor blood vessels, thereby increasing drug accumulation in the target tissue.6, 7 In fact, applying mild hyperthermia in vivo has been reported to increase blood flow within tumor tissues by up to three‐fold and the antitumor effects of drugs by up to 3.6‐fold.8
Cetuximab, an epidermal growth factor receptor (EGFR)‐targeted antibody, is a promising candidate for clinical applications in pancreatic cancer patients because EGFR is highly expressed in pancreatic cancer cells at frequencies of 60–90%.9 However, treating pancreatic cancer patients with a combination of gemcitabine and cetuximab did not improve patient prognosis compared with treatment with gemcitabine alone Median survival time (MST) of 5.9–6.3 months,10 most likely because cetuximab was not adequately delivered to the pancreatic cancer cells following simple i.v. administration.
We therefore reviewed the potential for combining mAb drug treatment with mild hyperthermia to deliver effective amounts of cetuximab to matrix‐rich pancreatic cancer. Reports from as early as the 1990s showed enhanced accumulation and an improved antitumor effect when radioimmunotherapy was used in combination with mild hyperthermia, although the booster effect still did not yield satisfactory outcomes.11, 12
The goal of this study was to determine whether mild hyperthermia could provide a booster effect for cetuximab therapy. Using a mouse model of s.c. pancreatic cancer, we provide preclinical evidence that mild hyperthermia enhances cetuximab accumulation and reduces tumor size.
Materials and Methods
Four in vivo pancreatic cancer models with different amounts of stroma
We used three pancreatic cancer cell line‐based xenograft models. The human pancreatic cancer cell lines MIAPaCa‐2 (CRL‐1420), BxPC‐3 (CRL‐1687), and Capan‐1 (HTB‐79) were purchased from ATCC (Manassas, VA, USA). The amounts of induced stroma are different between these cell lines: the stroma is abundant in Capan‐1 (++), modest in BxPC‐3 (+), and very scarce in MIAPaCa‐2 (±).13 To test a more stroma‐rich model, we also used a patient‐derived tumor fragment transplantation model (Ope‐xeno, ++).
The primary generation of Ope‐xeno models was established by inoculating 2 × 2 × 2‐mm pieces of surgically resected tissue fragments from human pancreatic cancer patients into the backs of mice with 100 μL Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). The use of the Ope‐xeno model was approved by the Ethics Committee of Tsukuba University Hospital (Tsukuba, Japan). When the implanted pieces generated tumors of 1 cm in diameter, they were harvested and cut into 2 × 2 × 2‐mm pieces and transplanted into other mice. When these second generation tumors grew, they were minced into 2 × 2 × 2‐mm pieces and preserved in a freezer after soaking in cell cryopreservation medium (Cell Banker 1; Juji Field, Tokyo, Japan). The Ope‐xeno model in this study was designated the third generation, which was confirmed to maintain the histology of the original surgical sample even after the freeze–thaw process and to provide dense stromal proliferation.13
Epidermal growth factor receptor expression was assessed by immunohistochemistry.14 The MIAPaCa‐2 Cell‐xeno model and the Ope‐xeno model showed high levels of EGFR expression, whereas the BxPC‐3 and Capan‐1 Cell‐xeno models showed moderate expression levels (Fig. 1).15
Figure 1.
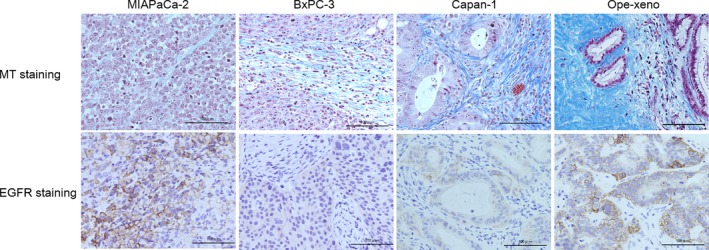
Four in vivo pancreatic cancer cell xenograft mouse models with different stroma amounts were used (scarce, MIAPaCa‐2; moderate, BxPC‐3; and abundant, Capan‐1 and Ope‐xeno). The epidermal growth factor receptor (EGFR) expression level is very high in the MIAPaCa‐2 Cell‐xeno and Ope‐xeno models and is moderate in the BxPC‐3 and Capan‐1 Cell‐xeno models. MT, Masson trichrome.
In vivo hyperthermia model using the water bath method
We used the water bath method to apply heat to the mouse tumors.16 The femoral region of the right hind leg of the mouse was used as the inoculation site. For water bath heating, the mice were anesthetized with inhaled isoflurane and positioned in specially designed holders that allowed the isolated leg tumor to be placed in the water bath.
Nude mice (8‐week‐old female, BALB/cA‐nude; CLEA Japan, Tokyo, Japan) were used for the experiments. We used 100 μL PBS as a buffer for the 5 × 106 cancer cells that were inoculated in the Cell‐xeno models. We prepared human pancreatic cancer cells using RPMI‐1640 medium (Sigma‐Aldrich, St. Louis, MO, USA) for Capan‐1 and BxPC‐3 cells and DMEM (Sigma‐Aldrich) for MIAPaCa‐2 cells; the media were supplemented with 10% FBS.
Study 1: tumor growth inhibition by cetuximab and the booster effect provided by hyperthermia
When the mouse leg tumor volume reached 70 mm3, 0.1 mg/kg cetuximab (Merck, Darmstadt, Germany) in 100 μL normal saline was systemically administered through the caudal vein.15, 17, 18 We used normal saline as a solution buffer for cetuximab. The tumors were then warmed in a water bath for 30 min at different temperatures: 25°C, control temperature; 37°C, intra‐abdominal organ level; and 41°C, mild hyperthermia (n = 4, each treatment group).6, 19 The cetuximab plus heat treatment was given once every 3 days for a total of 10 applications (Fig. 2).18 The control group received a normal saline injection plus heat (n = 4). The size of each xenograft was determined by measuring the tumor diameter and calculating the tumor volume (V) using the following formula: V = πab 2/6 where a is the maximum diameter and b is the minimum diameter.
Figure 2.
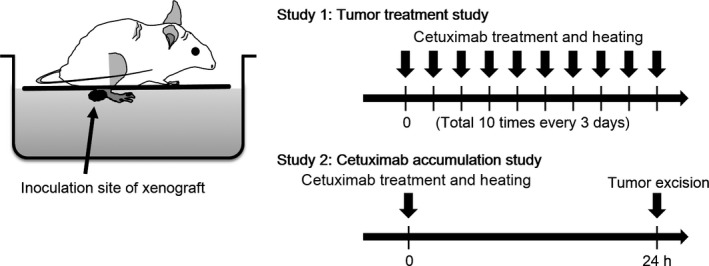
In vivo hyperthermia model. Study 1, antitumor effects were determined by measuring tumor volume over time. Study 2, cetuximab accumulation in tumor cells was quantified by immunofluorescence.
Study 2: quantification of in vivo accumulation of cetuximab in cancer cells and effect of hyperthermia
To objectively determine the amount of hyperthermia‐enhanced cetuximab accumulation in vivo in a single cancer cell, we used an immunofluorescence technique using anti‐human IgG antibodies that recognized the Fc portion of cetuximab, which were visualized using a fluorescence dye. The mice in all four groups were treated with cetuximab + different temperatures (25, 37, or 41°C, n = 4 each; i.e., four groups × three temperatures) using the same protocol described in Study 1 but for one total session. At 24 h after the treatment, the mice were killed by cervical dislocation, the tumors were excised, and cetuximab accumulation was measured (Fig. 2). The excised tumors were fixed in formalin and sectioned into 2‐μm slices, and biotin was blocked using a biotin‐blocking system (Dako Japan, Tokyo, Japan). The sections were then incubated with an HRP‐conjugated polyclonal rabbit anti‐human IgG antibody (Dako, Glostrup, Denmark) at a 1:100 dilution for 24 h at 4°C, followed by incubation with a biotin‐conjugated tyramide signal amplification system (PerkinElmer, Waltham, MA, USA) at a 1:50 dilution for 10 min at room temperature. The tissues were then treated with streptavidin‐conjugated Alexa Fluor 488 dye (Alexa 488) (Life Technologies, Grand Island, NY, USA) at a 1:500 dilution for 30 min at room temperature, followed by counterstaining with DAPI (Life Technologies).
The single cell fluorescence intensity was quantified using a digital microscope (BZ‐9000; Keyence, Osaka Japan). A total of 10 sites, ranging from approximately 100 × 100 to 200 × 500 μm2, was selected for each section at 200× magnification, and the total Alexa 488 intensity was divided by the number of cancer cell nuclei (Fig. 3). When selecting the 10 sites, the stromal regions were excluded by manually selecting areas of cancer cells. The cetuximab accumulation per cancer cell in a section was defined as the median value of the 10 sites. The mean value for four mice was recorded.
Figure 3.
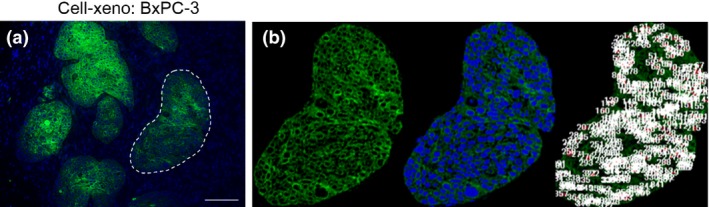
(a) Alexa 488 immunofluorescence staining in the BxPC‐3 Cell‐xeno pancreatic cancer xenograft mouse model. Only the target regions of the cancer cell aggregates were counted (indicated by outlining with a dashed line). (b) Manually selected cancer cell areas with an island‐shaped morphology (left), DAPI nuclear staining (middle), and cell counting by digital microscopy (right) in selected cancer cell areas. The sum of the Alexa 488 fluorescence intensity in cancer cells was divided by the number of cancer cells to indirectly quantify cetuximab accumulation per cancer cell.
Statistical analyses
All of the statistical analyses were carried out using spss‐ii for Windows (IBM, Armonk, NY, USA), and the data are presented as the mean ± SD. The F‐test was used to account for population variance, and Student's t‐test and the Tukey–Kramer test were used to compare two groups and multiple groups, respectively. P‐values <0.05 were considered to be statistically significant. The error bars in the figures represent the SD.
Results
Study 1: tumor growth inhibition by cetuximab and booster effect provided by hyperthermia
In the MIAPaCa‐2 Cell‐xeno model, cetuximab showed effective antitumor activity at 25°C (Fig. 4a) (●, 123 ± 30 mm3) compared with the Ns + 25°C group (○, 1462 ± 790 mm3). There was no apparent increase in the antitumor effect of cetuximab at 37°C (Fig. 4a) (▲, 109 ± 36 mm3; P = 0.25) or 41°C (■, 151 ± 10 mm3; P = 0.31) at day 50. Hyperthermia alone was not effective, as the average tumor sizes at 37°C (▵, 1612 ± 364 mm3) and 41°C (□, 1657 ± 1218 mm3) did not differ from those of the 25°C group (P = 0.33, 0.41) (Fig. 4a). Similar negative effects of heat were confirmed in the other three models.
Figure 4.
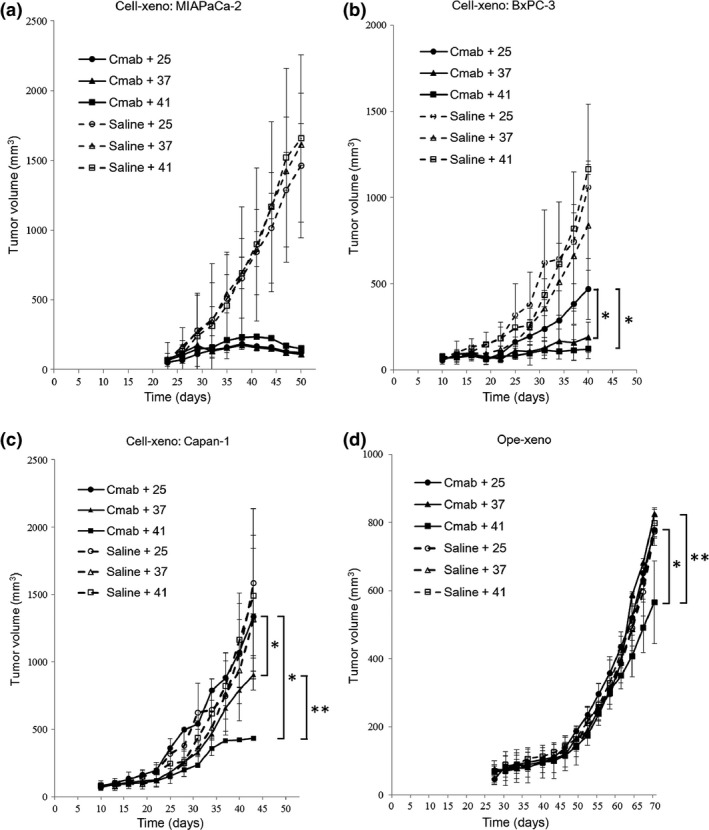
Tumor growth curves in four in vivo pancreatic cancer xenograft mouse models with different stroma amounts treated with cetuximab at different temperatures. (a) Mean MIAPaCa‐2 Cell‐xeno volume at day 50 was not significantly different in the cetuximab (Cmab) + 37°C group (▲, 109 ± 36 mm3) or the 41°C group (■, 151 ± 10 mm3) compared with the 25°C group (●, 123 ± 30 mm3). In the normal saline control groups, the mean tumor volume was not significantly different among the four xenograft models. (b) Mean BxPC‐3 Cell‐xeno volume at day 40 was significantly smaller in the cetuximab + 37°C group (▲, 189 ± 228 mm3) and the 41°C group (■, 121 ± 310 mm3) compared with the 25°C group (●, 470 ± 244 mm3). *P < 0.05, Cmab + 37 group and Cmab + 41 group versus Cmab + 25 group. (c) Mean Capan‐1 Cell‐xeno volume at day 40 was significantly smaller in the cetuximab + 37°C group (▲, 789 ± 36 mm3) and the 41°C group (■, 421 ± 14 mm3) compared with the 25°C group (●, 1070 ± 20 mm3). Furthermore, the mean tumor volumes were significantly smaller in the cetuximab + 41°C group (■, 421 ± 14 mm3) compared with the 37°C group (▲, 789 ± 36 mm3). *P < 0.05, Cmab + 37 group and Cmab + 41 group versus Cmab + 25 group. **P < 0.05, Cmab + 41 group versus Cmab + 37 group. (d) Mean Ope‐xeno volume at day 70 was significantly smaller in the Cetuximab + 41°C group (■, 565 ± 120 mm3) compared with the 37°C group (▲, 823 ± 38 mm3) and the 25°C group (●, 778 ± 38 mm3). *P < 0.05, Cmab + 41 group versus Cmab + 25 group. **P < 0.05, Cmab + 41 group versus Cmab + 37 group.
In the BxPC‐3 Cell‐xeno model, an intermediate antitumor effect of cetuximab was observed at 25°C (Fig. 4b) (●, 470 ± 244 mm3) compared with the Ns + 25°C group (○, 1059 ± 1076 mm3). This effect was enhanced by combining cetuximab treatment with hyperthermia, as demonstrated at 37°C (▲, 189 ± 228 mm3) and 41°C (■, 121 ± 310 mm3) at day 40 (all P < 0.05).
In the Capan‐1 Cell‐xeno model, no antitumor effect of cetuximab was observed at 25°C (Fig. 4c) (●, 1070 ± 20 mm3) compared with the Ns + 25°C group (○, 1059 ± 394 mm3). Hyperthermia enhanced the antitumor effect of cetuximab, as significant suppression of tumor growth was observed at 37°C (▲, 789 ± 36 mm3) and 41°C (■, 421 ± 14 mm3) compared with the 25°C group at day 40 (all P < 0.05). Furthermore, 41°C treatment further enhanced the cetuximab antitumor effect compared with 37°C treatment at day 40 (P < 0.05).
In the Ope‐xeno model, no antitumor effect of cetuximab was observed at 25°C (●, 778 ± 38 mm3) compared with the Ns + 25°C group (○, 774 ± 82 mm3). Hyperthermia only enhanced the antitumor effect of cetuximab at 41°C (■, 565 ± 120 mm3) at day 70 (P < 0.05) (Fig. 4d).
Study 2: quantification of accumulated cetuximab in a cancer cell and the effect of hyperthermia
The cetuximab accumulation in MIAPaCa‐2 cancer cells was sufficiently intense at 25°C (Fig. 5a‐i) and did not increase at 37°C or 41°C (Fig. 5a‐ii,a‐iii). The intensity value at 25°C (1632 ± 960; Fig. 5a‐iv, white column) was not increased at 37°C (1921 ± 848, gray column) or 41°C (1949 ± 690, black column) (P = 0.34 and 0.23, respectively).
Figure 5.
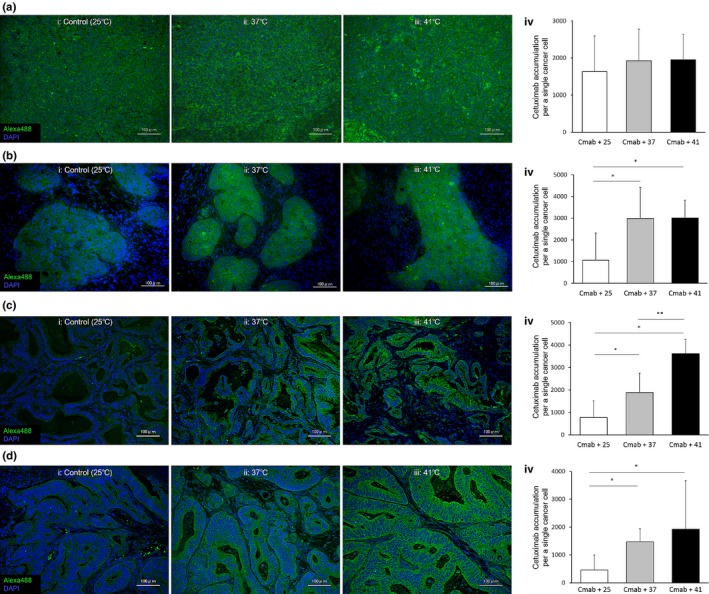
Cetuximab accumulation in cancer cells in four in vivo pancreatic cancer xenograft mouse models with different stroma amounts. (a) MIAPaCa‐2 Cell‐xeno model. The intensity values in the cetuximab + 25°C group (iv, white column) did not increase further with treatment at 37°C (gray column) or 41°C (black column). (b) BxPC‐3 Cell‐xeno model. The intensity values were significantly increased in the 37°C (iv, gray column) and 41°C (black column) groups compared with the cetuximab (Cmab + 25°C group (white column). *P < 0.05. (c) Capan‐1 Cell‐xeno model. The intensity values in the 37°C (iv, gray column) and 41°C (black column) groups were significantly increased compared with the cetuximab + 25°C group (white column). Furthermore, the Alexa 488 intensity values in the 41°C group (black column) were higher than those in the cetuximab + 37°C group (gray column). *P < 0.05. **P < 0.05. (d) Ope‐xeno model. The intensity values were significantly higher in the 37°C (iv, gray column) and 41°C (black column) groups compared with the cetuximab + 25°C group (white column). *P < 0.05.
The accumulation in BxPC‐3 cancer cells was moderate at 25°C (Fig. 5b‐i) and increased at 37 and 41°C (Fig. 5b‐ii,b‐iii). The intensity value at 25°C (1063 ± 1260; Fig. 5b‐iv, white column) was increased at 37°C (2980 ± 1438, gray column) and 41°C (3015 ± 816, black column) (all P < 0.05).
The accumulation in Capan‐1 and Ope‐xeno cancer cells was very low at 25°C (Fig. 5c‐i,d‐i) and increased at 37 and 41°C (Fig. 5c‐ii,c‐iii,d‐ii,d‐iii). The intensity values at 25°C (760 ± 756, 461 ± 536; Fig. 5c‐iv,d‐iv, white column) were increased at 37°C (1881 ± 870, 1469 ± 472, gray column) and 41°C (3615 ± 634, 1922 ± 1740, black column) (all P < 0.05). Furthermore, in the Capan‐1 Cell‐xeno model, the intensity value at 41°C was higher than that at 37°C (P < 0.05).
Discussion
The effects of mild hyperthermia on antibody therapy were determined in the 1990s for radioimmunotherapy using stroma‐poor colon cancer mouse models.11, 12 Although the effectiveness of radioimmunotherapy alone has been insufficient, research in this field advanced very little in the subsequent 20 years. By combining mild hyperthermia with cetuximab treatment, we showed drug accumulation in tumors and potent antitumor effects in an in vivo model of stroma‐rich pancreatic cancer.
Optimal cetuximab effects were obtained in the stroma‐poor MIAPaCa‐2 Cell‐xeno model, which showed complete remission without the addition of heat treatment (Fig. 4a). This effect may be attributed to sufficient in vivo accumulation of cetuximab in the cancer cells, as demonstrated by the fluorescence intensity value of 1632/cell (Fig. 5a). Because cetuximab treatment without heat (i.e., at 25°C) was sufficient in the MIAPaCa‐2 Cell‐xeno model, we confirmed that there was no need for the additive effect of mild hyperthermia.
Stroma is viewed as a hindrance to drug delivery.4, 20 In fact, cancer cells are separated from the nearest blood vessels by prominent stromal fibers, and the distances between cancer cells and blood vessels are significantly longer in human pancreatic cancer (47.4 ± 43.0 μm) and in the Ope‐xeno model (35.3 ± 39.0 μm) than in the stroma‐poor cell xenograft models (3.9 ± 3.1 μm).13 In addition, the vessel density of stroma‐rich tumors is lower than that of stroma‐poor tumors.13 In terms of the relationship between tumor stroma and hyperthermia, we assumed that stroma‐rich cancer would be a better candidate for the booster effect of hyperthermia.
First, we assumed that applying hyperthermia would affect physiological phenomena in the tumor stroma, which includes fibroblasts and endothelial cells. In fact, a previous in vitro study showed that the colony formation of fibroblasts and endothelial cells, which play fundamental roles in tumor stroma, was maximally reduced to 17% and 5%, respectively, in response to hyperthermia.21 Furthermore, previous in vivo studies reported that treating tumors with hyperthermia improved tumor vessel function and increased the pore size of tumor vessels, thus increasing anticancer drug extravasation.21, 22 These findings indicated that hyperthermia could improve drug delivery by reducing the stromal barrier or increasing anticancer drug extravasation.
Second, we assumed that hyperthermia would improve drug delivery by increasing tumor blood flow. Stroma‐rich tumors are largely hypovascular; therefore, there is less blood supply per unit of tumor weight than in stroma‐poor and hypervascular tumors. We assumed that the blood flow increase after heating compared with before heating would be more pronounced in stroma‐rich cancer. The number of vessels, the resultant hypoxia, and the low pH of the intratumor environment are recognized hallmarks of stroma‐rich tumors that evoke resistance to anticancer drugs and radiation.4, 20 After application of mild hyperthermia, the oxygen content and pH of this stroma‐rich tumor environment increase, rendering the tumor more susceptible to anticancer drugs and radiation.6, 7, 8
The abundance of the tumor stroma is closely related to the effect of cetuximab plus mild hyperthermia treatment. The general explanation for this phenomenon is that tumors with more abundant stroma accumulate less cetuximab, and thus, tumor growth is not inhibited under normal conditions (i.e., at 25°C). In fact, a moderate antitumor effect was observed in the BxPC‐3 Cell‐xeno (moderate stroma) model at 25°C; however, this effect was weaker than that observed in the stroma‐poor MIAPaCa‐2 Cell‐xeno model (Fig. 4b). In the stroma‐rich Capan‐1 Cell‐xeno and Ope‐xeno models, we observed no effect at 25°C. With the application of mild heat (37°C), the antitumor effects increased in the BxPC‐3 Cell‐xeno model and were significantly increased in the Capan‐1 model (Fig. 4b,c). Moreover, when the temperature was increased to 41°C, the tumors in the BxPC‐3 Cell‐xeno model were completely eradicated, the effectiveness of cetuximab in the Capan‐1 model increased, and we detected an effect in the stroma‐rich Ope‐xeno model (Fig. 4b–d). Of note, the additive effect of mild hyperthermia appeared to be more significant in stroma‐rich cancers, such as the Capan‐1 Cell‐xeno model.
The antitumor booster effect of mild hyperthermia may be due to increased cetuximab accumulation, as measured by fluorescence intensity values. The baseline accumulation (i.e., at 25°C without heat treatment) of cetuximab was inversely proportional to the amount of stroma present (Fig. 5a‐iv–d‐iv, white columns), and the values detected in the moderate to rich stroma models were far below the benchmark value of approximately 1600–2000/cell in the MIAPaCa‐2 Cell‐xeno model. Applying 37°C heat increased these values by 2.8‐, 2.5‐, and 3.2‐fold in the BxPC‐3, Capan‐1 Cell‐xeno and Ope‐xeno models, respectively, achieving values that were comparable to the baseline value in the MIAPaCa‐2 Cell‐xeno model of 1600–2000/cell. Moreover, in the stroma‐rich Capan‐1 Cell‐xeno model, treatment with 41°C heat resulted in an increased booster effect of up to 4.8‐fold compared with the baseline value of 760/cell at 25°C.
Thus, the question arises as to why applying heat has such a considerable effect. One explanation is that mild hyperthermia increases blood flow in the tumor and enhances tumor vessel permeability, thereby promoting cetuximab delivery to the target tumor and enhancing the antitumor effect. The mechanisms underlying the hyperthermia‐induced increase in tumor blood flow and tumor vessel permeability have been previously reported.7, 23, 24, 25 In terms of the heating period, Song et al. previously reported that the blood flow in mammary adenocarcinoma R3230 tumors increased approximately 1.5‐fold immediately after heating at 40.5°C or 41.58°C for 30–60 min, whereas it increased 2.5‐fold 24 h after heating at 41.58°C for 60 min.7, 23, 24, 25 Therefore, we think that the hyperpermeability of tumor vasculature is maximally enhanced by mild hyperthermia at 24 h after heating.
Tumor blood vessels dilate in response to mildly elevated temperatures, most likely due to smooth muscle relaxation through stimulation by nitric oxide synthesized by endothelial cells.23 Subsequently, blood enters the dilated arterioles in the tumor and floods the network of capillary‐like tumor blood vessels, increasing the intravascular pressure and flow rate.7 Furthermore, previous in vivo studies have shown that increased hyperthermia‐induced blood flow is caused by vessel dilation and the recruitment of capillaries following stimulation with vasoactive compounds such as bradykinin and/or histamine.24 Regarding the mechanisms underlying hyperthermia‐enhanced tumor vessel permeability, the efficacy of most chemotherapeutics is usually predicated on achieving adequate drug transport across the endothelial layer and into the tumor interstitial stroma. The gaps within the endothelial lining are an indication of the stress induced on the tumor vasculature by mild hyperthermia. In response to thermal stress, the loosening or creation of gaps between endothelial cells in tumor vessels improves stromal delivery.25
Another possible explanation for the enhanced anticancer effect of cetuximab combined with mild hyperthermia is an increase in the antigen–antibody interaction between cetuximab and EGFR, which is known to be affected by temperature. In fact, Johnstone et al. assessed the effects of temperature over a range of 1–37°C and reported a 2‐ to 18‐fold increase in the association rate and a 20‐ to 50‐fold increase in the dissociation rate of antibodies to and from murine cell surface antigens, respectively.26 However, the temperature range used for mild hyperthermia, that is, heating from a baseline of 37–45°C, did not affect the association or dissociation rates of the mAb binding.27 We now understand that altering the interaction kinetics of mAbs does not play a role in the hyperthermia‐induced enhancement of mAb targeting to cancer cells.
Tumors that carry k‐ras mutations, such as pancreatic cancer, are not candidates for anti‐EGFR antibody drug therapy.28, 29, 30 However, our data indicate that cetuximab is effective in pancreatic cancers with the k‐ras mutation, such as the MIAPaCa‐2 and Capan‐1 cell lines,30, 31 if a sufficient amount of the antibody can be delivered to and accumulate at the target site. Moreover, in vivo and in vitro studies that used EGFR knockdown to suppress the proliferation of a pancreatic cancer cell line carrying the k‐ras mutation and a clinical study of anti‐EGFR antibody drugs in patients with pancreatic cancer showed no differences in outcome related to k‐ras status.30, 32 Although the majority (>90%) of clinical pancreatic cancers are positive for k‐ras mutation, cetuximab may be effective if used in combination with mild hyperthermia.
The majority of studies investigating mild hyperthermia have conventionally been carried out in settings comparing 25 and 41°C, likely because mild hyperthermia is primarily used to target body surface cancers such as those of the skin or neck.5, 33 However, if we intend to treat i.p. tumors such as pancreatic cancer, which occur in a 37°C environment, then comparisons between heat treatment at 37 and 41°C are indispensable. Because such studies have largely been lacking, the results of the present study provide valuable data showing that cetuximab accumulation and the resulting antitumor effects are enhanced by treatment at 41°C compared with 37°C, especially in the stroma‐rich Capan‐1 Cell‐xeno and Ope‐xeno models. Fortunately, microwave or radiofrequency energy applicators for delivering mild hyperthermia, such as the Thermotron RF‐8 (Yamamoto Vinita, Osaka, Japan), can be used clinically to treat advanced i.p. tumors.6 Consequently, combining cetuximab and mild hyperthermia may be a realistic option to consider for the clinical treatment of pancreatic cancer patients.
Disclosure Statement
The authors have no conflict of interest.
Cancer Sci 107 (2016) 514–520
Funding Information
This study was supported in part by Grant‐in‐Aid for Scientific Research (KAKENHI, 20282353 and 23300185) from The Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Warshaw AL, Fernandez‐del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–65. [DOI] [PubMed] [Google Scholar]
- 3. Olive KP, Jacobetz MA, Davidson CJ et al Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324: 1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olson P, Hanahan D. Cancer. Breaching the cancer fortress. Science 2009; 324: 1400–1. [DOI] [PubMed] [Google Scholar]
- 5. Skibba JL, Jones FE, Condon RE. Altered hepatic disposition of doxorubicin in the perfused rat liver at hyperthermic temperatures. Cancer Treat Rep 1982; 66: 1357–63. [PubMed] [Google Scholar]
- 6. van der Zee J. Heating the patient: a promising approach? Ann Oncol 2002; 13: 1173–84. [DOI] [PubMed] [Google Scholar]
- 7. Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21: 761–7. [DOI] [PubMed] [Google Scholar]
- 8. Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia 1999; 15: 79–107. [DOI] [PubMed] [Google Scholar]
- 9. Fujita H, Ohuchida K, Mizumoto K et al High EGFR mRNA expression is a prognostic factor for reduced survival in pancreatic cancer after gemcitabine‐based adjuvant chemotherapy. Int J Oncol 2011; 38: 629–41. [DOI] [PubMed] [Google Scholar]
- 10. Philip PA, Benedetti J, Corless CL et al Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group‐directed intergroup trial S0205. J Clin Oncol 2010; 28: 3605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kinuya S, Yokoyama K, Hiramatsu T et al Combination radioimmunotherapy with local hyperthermia: increased delivery of radioimmunoconjugate by vascular effect and its retention by increased antigen expression in colon cancer xenografts. Cancer Lett 1999; 140: 209–18. [DOI] [PubMed] [Google Scholar]
- 12. Wilder RB, Langmuir VK, Mendonca HL, Goris ML, Knox SJ. Local hyperthermia and SR 4233 enhance the antitumor effects of radioimmunotherapy in nude mice with human colonic adenocarcinoma xenografts. Cancer Res 1993; 53: 3022–7. [PubMed] [Google Scholar]
- 13. Akashi Y, Oda T, Ohara Y et al Histological advantages of the tumor graft: a murine model involving transplantation of human pancreatic cancer tissue fragments. Pancreas 2013; 42: 1275–82. [DOI] [PubMed] [Google Scholar]
- 14. Atkins D, Reiffen KA, Tegtmeier CL, Winther H, Bonato MS, Storkel S. Immunohistochemical detection of EGFR in paraffin‐embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem 2004; 52: 893–901. [DOI] [PubMed] [Google Scholar]
- 15. Overholser JP, Prewett MC, Hooper AT, Waksal HW, Hicklin DJ. Epidermal growth factor receptor blockade by antibody IMC‐C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer 2000; 89: 74–82. [PubMed] [Google Scholar]
- 16. Wiedemann GJ, Siemens HJ, Mentzel M et al Effects of temperature on the therapeutic efficacy and pharmacokinetics of ifosfamide. Cancer Res 1993; 53: 4268–72. [PubMed] [Google Scholar]
- 17. Luo FR, Yang Z, Dong H et al Correlation of pharmacokinetics with the antitumor activity of Cetuximab in nude mice bearing the GEO human colon carcinoma xenograft. Cancer Chemother Pharmacol 2005; 56: 455–64. [DOI] [PubMed] [Google Scholar]
- 18. Wild R, Fager K, Flefleh C et al Cetuximab preclinical antitumor activity (monotherapy and combination based) is not predicted by relative total or activated epidermal growth factor receptor tumor expression levels. Mol Cancer Ther 2006; 5: 104–13. [DOI] [PubMed] [Google Scholar]
- 19. Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008; 44: 2546–54. [DOI] [PubMed] [Google Scholar]
- 20. Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006; 6: 583–92. [DOI] [PubMed] [Google Scholar]
- 21. Dings RP, Loren ML, Zhang Y et al Tumour thermotolerance, a physiological phenomenon involving vessel normalisation. Int J Hyperthermia 2011; 27: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kong G, Anyarambhatla G, Petros WP et al Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 2000; 60: 6950–7. [PubMed] [Google Scholar]
- 23. Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155: 515–28. [DOI] [PubMed] [Google Scholar]
- 24. Song CW. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res 1984; 44: 4721s–30s. [PubMed] [Google Scholar]
- 25. Li L, ten Hagen TL, Bolkestein M et al Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release 2013; 167: 130–7. [DOI] [PubMed] [Google Scholar]
- 26. Johnstone RW, Andrew SM, Hogarth MP, Pietersz GA, McKenzie IF. The effect of temperature on the binding kinetics and equilibrium constants of monoclonal antibodies to cell surface antigens. Mol Immunol 1990; 27: 327–33. [DOI] [PubMed] [Google Scholar]
- 27. Hauck ML, Dewhirst MW, Zalutsky MR. The effects of clinically relevant hyperthermic temperatures on the kinetic binding parameters of a monoclonal antibody. Nucl Med Biol 1996; 23: 551–7. [DOI] [PubMed] [Google Scholar]
- 28. Deramaudt T, Rustgi AK. Mutant KRAS in the initiation of pancreatic cancer. Biochim Biophys Acta 2005; 1756: 97–101. [DOI] [PubMed] [Google Scholar]
- 29. Loupakis F, Cremolini C, Masi G et al Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014; 371: 1609–18. [DOI] [PubMed] [Google Scholar]
- 30. Navas C, Hernandez‐Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k‐ras oncogene‐driven pancreatic ductal adenocarcinoma. Cancer Cell 2012; 22: 318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ardito CM, Gruner BM, Takeuchi KK et al EGF receptor is required for KRAS‐induced pancreatic tumorigenesis. Cancer Cell 2012; 22: 304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kullmann F, Hartmann A, Stohr R et al KRAS mutation in metastatic pancreatic ductal adenocarcinoma: results of a multicenter phase II study evaluating efficacy of cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first‐line therapy. Oncology 2011; 81: 3–8. [DOI] [PubMed] [Google Scholar]
- 33. Dahl O. Interaction of hyperthermia and chemotherapy. Recent Results Cancer Res 1988; 107: 157–69. [DOI] [PubMed] [Google Scholar]


