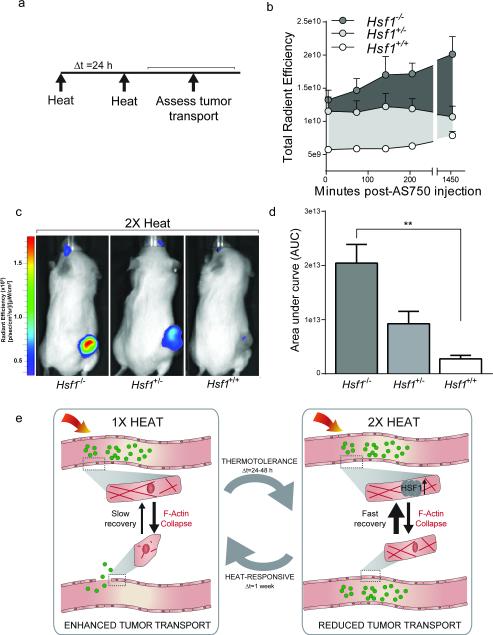
Figure 7.
Genetic ablation of HSR prevents vascular thermotolerance in response to repeated PEG-NR heating. (a) Ovarian tumor xenografts established in either homozygous-null (−/−), heterozygous (+/−), or wild type (+/+) Hsf1 animals received an initial PEG-NR heat exposure (heat), an interval delta T of 24 h, and a re-exposure to PEG-NR heating (heat). Tumor transport was assayed by intravenous injection of AS750 post-heating, followed by serial measurements of AS750 fluorescence for 24 h by in vivo fluorescence imaging. (b) Tumor accumulation of transported AS750 for each genetic background up to 24 h after treatment. (n = 3-6 per group) Error bars, SE. (c) Representative in vivo fluorescence images in animals with Hsf1−/−, Hsf1+/−, and Hsf1+/+ backgrounds receiving double PEG-NR heat exposures. (d) Area under the curve from panel (b) for each genetic background demonstrating enhanced accumulation in Hsf1−/− animals. (n = 3-6 per group, **P<0.01, one-way ANOVA and Tukey's post-tests.) Error bars, SE. (e) Proposed model for the effects of PEG-NR heating on tumor transport and acquired thermotolerance. An initial exposure to PEG-NR heating alters the endothelial architecture via cytoskeletal collapse, leading to enhanced tumor transport of nanoparticle cargos. Over the next 24-48 h, acquired thermotolerance, mediated by the heat-shock response and Hsf1, upon re-exposure to PEG-NR heating, results in enhanced cytoskeletal recovery and diminished tumor transport. Restoration of heat-sensitivity occurs after approximately 1 week from the initial heat exposure.