Abstract
The degradation of phosphorothioate oligonucleotides (PS-ONDs) and the release of potentially genotoxic modified mononucleotides raise a safety concern for OND-based therapeutics. Deoxyadenosine monophosphorothioate (dAMPαS), a PS nucleotide analog, has been reported to be a potent in vitro mutagen at the thymidine kinase (TK) locus in human TK6 lymphoblastoid cells. This led us to explore the mechanism behind the apparent positive response induced by dAMPαS in the TK gene-mutation assay in TK6 cells. In this work, treatment of TK6 cells with dAMPαS produced a dose-dependent increase in cytotoxicity and mutant frequency at the TK locus. Surprisingly, when the colonies from dAMPαS were re-challenged with the selective agent trifluorothymidine (TFT), the TFT-resistant phenotype was lost. Moreover, dAMPαS-induced colonies displayed distinct growth kinetics and required longer incubation time than 4-nitroquinoline-1-oxide-induced colonies to start growing. Treatment of TK6 cells with dAMPαS induced cell cycle arrest at the G1 phase, enabling cells to grow, and form a colony after the efficacy of TFT in the culture medium was lost. Our findings suggest that a fraction of parental “nonmutant” TK6 cells escaped the toxicity of TFT, possibly via G1 arrest, and resumed growth after the degradation of TFT. We conclude that dAMPαS did not induce real TFT-resistant mutants and caution should be taken with interpretation of mutation data from TK gene-mutation assay in TK6 cells when assessing modified nucleotides.
Keywords: nucleotide, phosphorothioate, thymidine kinase, mutagenesis
Oligonucleotide (OND)-based therapeutics and antisense ONDs in particular, are widely used for modulating gene expression inside cells with promising clinical potential (Graham et al., 2013; Thomas et al., 2013). Various chemical modifications to the native ONDs structures including the backbone, sugar, and/or base have been developed to improve their therapeutic potential (Deleavey and Damha, 2012). Chemically modified ONDs, in which one of the nonbridging oxygen atoms of the phosphodiester backbone is replaced with a sulphur atom, are commonly utilized in antisense applications with several candidates currently explored in clinical trials. This phosphorothioate (PS) substitution increases the nuclease resistance of PS-ONDs while maintaining target mRNA binding and degradation by intracellular ribonuclease H (RNase H) (Eckstein, 2000). Following systemic administration, PS-ONDs are rapidly cleared from the blood and distributed into all tissues with the kidney and the liver showing the highest accumulation (Geary et al., 1997). Clearance from tissues results mainly from the exonuclease metabolism of PS-ONDs into shortened ONDs and the release of mononucleotide analogs. These mononucleotides could potentially enter and disrupt the endogenous nucleotide pools, which are known to have genotoxic consequences, including mutations (Anderson et al., 1981; Mattano et al., 1990), DNA strand breaks (Brox et al., 1984), and chromosomal abnormalities (Ryan et al., 1965). However, perhaps of more concern, the liberated nucleotide analogs could be a substrate for various kinases and become incorporated into the genomic DNA during synthesis. If base pairing with the nucleotide analog occurred with reduced fidelity, a mutagenic event may arise http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003149.pdf; Fersht, 1979).
Recently, it has been reported that a PS nucleotide analog, deoxyadenosine monophosphorothioate (dAMPαS), had a potent mutagenic response at the endogenous thymidine kinase (TK) gene in human lymphoblastoid TK6 cells (Reshat et al., 2012). In a different study, Liber et al. (1985) likewise have reported that the nucleoside analog, 5-bromodeoxyuridine (BrdUrd), induces mutations at the TK locus, whereas no mutation was observed at the phosphoribosyl-transferase (HPRT) locus. However, because isolated BrdUrd-induced colonies were sensitive to the selective agent, trifluorothymidine (TFT), the authors concluded that BrdUrd produced a transient TFT-resistant phenotype that did not result from a mutagenic mechanism. It should be noted that BrdUrd, as a thymidine analog, has been utilized as a selective agent for TK mutants in L5178Y mouse lymphoma cells (Clive et al., 1972), and thus may exert selective toxicity to TK-competent cells resulting in the outgrowth of pre-existing TK mutants. In addition, two phenotypic classes of TK mutants are known to occur in the TK6 assay and are usually characterized as normal growing (NG), which are normally scored within 14 days, or slow growing (SG) mutants which require extended incubation time (Liber et al., 1989). However, the late appearing colonies isolated from BrdUrd-induced cultures were TFT-sensitive and thus are not actually mutants. This prompted us to explore further if dAMPαS could induce a similar unstable TFT-resistant phenotype and, if that was the case, to provide insight as to the mechanism of such effect. In this study, we characterized the TFT-resistant colonies induced by dAMPαS by assessing their ability to survive following TFT-rechallenge, and investigated the influence of dAMPαS on the cell cycle. We found that dAMPαS-induced colonies were SG and lost the TFT-resistant phenotype when re-challenged with TFT. Our studies indicate that cell cycle arrest and loss of TFT efficacy lead to the growth of non-mutant colonies.
MATERIALS AND METHODS
Cell culture
The TK6 cell line (known in early publications as H2BT) is a subclone of WI-L2 which was established in 1968 from the spleen of 5-year-old male having hereditary spherocytic anemia (Levy et al., 1968). The development of TK heterozygous TK6 cell line was obtained following treatment of human lymphoblast line HH4 with ICR191 (Skopek et al., 1978). The cells used for this study were obtained as a gift from Swansea University in 2009. In house karyotypic analysis in 2010 showed these TK6 cells to have a modal chromosome number of 47 and a stable composite karyotype of 47 XY, + der13t(13;22) −14 + der14t (14;20) der 21 (21,3) (Molloy et al., 2010) that matches the karyotype published by Honma (2005). TK6 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% heat-inactivated horse serum, 2 mM l-glutamine, 100 U/ml of penicillin, and 100 µg/ml of streptomycin. The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. All cell culture reagents were purchased from Invitrogen (Paisley, United Kingdom). Modified PS nucleotides (dAMPαS and dCMPαS) were obtained from Jena Bioscience, Germany. The unmodified nucleotide (dAMP) was purchased from Sigma-Aldrich (Poole, United Kingdom).
Treatment of cells with nucleotides
Treatment of TK6 cells with nucleotides was performed as previously described (Reshat et al., 2012). In brief, TK6 cells (4 × 106) were aliquoted per 75 cm2 flask in a 5-ml volume and were mixed with nucleotides dissolved in cell culture medium to a 2 × concentration (5 ml) for 24 h. As a positive control, 4-nitroquinoline-1-oxide (4-NQO, 0.33 µM) was used. Cells were then harvested by centrifugation at 200 × g for 5 min, washed with culture media, and resuspended in fresh culture medium (3 × 105 cell/ml).
TK and HPRT gene-mutation assay
After treatment, cells were cultured and counted for 3 days to determine relative suspension growth (RSG) which was calculated based on the percentage of viable cells in the treated culture relative to control culture. On the third day, TK6 cells were plated in duplicate for each treatment at 1.6 cells per well in 96-well plates in the absence of TFT to determine cloning efficiency, and at a density of 20 000 cells per well in a medium containing 4 µg/ml of TFT to select TK−/− mutants. Relative total growth (RTG) (the product of RSG and cloning efficiency in non-selective medium) was calculated and used to assess cytotoxicity. The remaining cells were allowed to grow for additional 4 days for the HPRT gene-mutation assay. The HPRT mutant frequency was determined by plating 20 000 cells per well in 96-well plates in a medium containing 5 µg/ml of 6-thioguanine (6-TG), and the cloning efficiency was determined by seeding 1.6 cells per well in the absence of 6-TG. After 3 weeks of incubation at 37°C in 5% CO2, colonies were scored and the mutation frequency was calculated based on Poisson distribution as described previously (Clements, 2000).
Stability of TFT-resistant phenotype
Isolated TFT-resistant colonies (induced or spontaneous, 10 in total) were isolated and allowed to grow in a fresh medium without TFT for 1 week. The cells were then tested for TFT resistance by cloning cells as described earlier.
The in vitro micronucleus assay
TK6 cells (3 × 105 cell/ml) were removed after 24 h of treatment, collected by centrifugation, and suspended in 2 ml of culture medium containing 0.2% pluronic F-68. Cells were then spun onto microscope slides in duplicate for each treatment. Slides were fixed in absolute methanol for 1 min, washed with phosphate-buffered saline (PBS), and incubated in acridine orange (0.12 mg/ml) for 1 min. After staining, the slides were washed twice for 10 min in PBS. Slides were visualized using the Leitz Diaplan fluorescence microscope. One thousand cells were evaluated for the presence of micronuclei per slide. Cytotoxicity of cells following treatment was expressed as relative population doubling (RPD) as described in OECD guideline 487.
Time course of colony formation
Following treatment of TK6 cells with the modified nucleotide, cells were plated at 20 000 cells/well in 96-well plates in the presence of TFT (4 µg/ml). Four ×4 phase-contrast time lapse images were taken by IncuCyte ZOOM every day over period of 3 weeks, and then processed with ImageJ.
Cell cycle analysis
TK6 cells were treated with the modified nucleotide for 24 h, washed, and allowed to grow for an additional 48 h. Cells were then washed and fixed in 70% ice-cold ethanol for 30 min and resuspended in PBS solution containing 50 µg/ml of propidium iodide and 200 µg/ml of RNase at 37°C for 30 min. The cells were then analyzed on a Becton Dickinson FACSCanto II flow cytometer, using FACDiva software.
Statistical analysis
Two-tailed Student’s t test was used for statistical analysis of data from cell cycle experiments and to compare the RTG, mutant frequency between different treatments in TK and HPRT gene mutation assays.
RESULTS
Cytotoxicity and Mutagenicity of PS Nucleotide Analog, dAMPαS
It was previously reported that dAMPαS induces a significant mutation at the TK locus in human TK6 cells (Reshat et al., 2012). To verify this positive effect, TK6 cells were treated with various concentrations of dAMPαS for 24 h and then assessed for mutation at the TK and HPRT loci. As a positive control, TK6 cells were treated with 0.33 µM of 4-NQO. As shown in Table 1, dAMPαS induced concentration-dependent cytotoxicity resulting in 6% RTG at the highest concentration (1.5 mM). No significant cytotoxicity was observed with the unmodified nucleotide (dAMP) up to 1 mM (p > .05). Treatment with dAMPαS (> 0.25 mM) resulted in a concentration-dependent increase in TFT-resistant mutant fraction (p < .01) but no increase in HPRT mutants was observed even at the highest concentration (1.5 mM). These results are in agreement with previous studies (Reshat et al., 2012). No positive response was observed with dAMP at the TK or the HPRT loci (p > .05). Treatment with 4-NQO resulted in approximately 7-fold and approximately 8-fold increase in the TK and HPRT mutant frequency, respectively. dAMPαS was also evaluated by the in vitro micronucleus assay. No clear concentration-related increase in micronuclei was observed following treatment with dAMPαS up to 1.5 mM (Table 1).
TABLE 1.
Genotoxicity and Cytotoxicity of dAMPαS Nucleotide Analog in Human TK6 Cells
| dAMPαS (mM) | RTG (%)a | Mutant frequency (× 10−6)a |
Micronucleus assaya |
||
|---|---|---|---|---|---|
| TK | HPRT | RPD (%) | % MNb | ||
| Control | 103.5 ± 9.2 | 10.8 ± 1.1 | 3.0 ± 0.1 | 99.6 ± 0.2 | 0.8 ± 0.8 |
| 0.25 | 93.5 ± 5.0 | 19.5 ± 3.5 | 3.2 ± 0.4 | 96.3 ± 1.2 | 1.0 ± 0.6 |
| 0.5 | 61.0 ± 2.8 | 114.0 ± 14.1** | 4.1 ± 0.7 | 87.4 ± 0.6 | 0.9 ± 0.7 |
| 0.75 | 35 ± 5.0 | 377.5 ± 3.6*** | 3.3 ± 0.3 | 80.4 ± 3.8 | 1.1 ± 0.5 |
| 1 | 19.0 ± 1.4 | 571 ± 51.6** | 4.0 ± 0.4 | 69.3 ± 2.7 | 0.9 ± 0.6 |
| 1.25 | 12.0 ± 0 | 609 ± 12.0*** | 2.5 ± 0.7 | 65.3 ± 3.0 | 1.2 ± 0.5 |
| 1.5 | 5.5 ± 0.7 | 1416.0 ± 69.3** | 2.5 ± 0.7 | 58.1 ± 0.1 | 1.3 ± 0.5 |
| dAMPc | 93 ± 2.8 | 9.3 ± 9.2 | NA | NA | NA |
| 4-NQOd | 25 ± 4.2 | 82.0 ± 5.6** | 23.5 ± 0.7*** | 55.2 ± 4.2 | 2.5 ± 0.1** |
aValues represent the mean ± SD of three independent experiments. **p < .01 and ***p < .001, as compared with untreated control.
bMN, micronuclei.
cdAMP, deoxyadenosine monophosphate (1 mM).
d4-NQO, 4-nitroquinoline-1-oxide (0.00033 mM).
Stability of the TFT-Resistant Phenotype
The stability of the TFT-resistant phenotype in colonies recovered from dAMPαS was assessed. A total of 10 colonies were isolated, allowed to grow in a medium without TFT, and then re-challenged with TFT. Only 1 of 10 colonies isolated from each of the dAMPαS treatments at 0.75, 1, and 1.25 mM, was found to be stable (Table 2). Moreover, none of the 10 colonies resulting from each of the 0.5 or 1.5 mM dAMPαS treatments were found to be TFT-resistant. In contrast, TFT resistance was observed in all 10 spontaneous TK mutant colonies as well as those isolated following 4-NQO exposure (Table 2). These results suggest that dAMPαS-induced colonies were temporarily TFT-resistant, indicating that they were not stable TK−/− mutants.
TABLE 2.
Stability of TFT-Resistant Phenotype in Colonies Isolated by TFT, dAMPαS, or 4-NQOa
| dAMPαS (mM) | Number of TFT-resistant colonies |
|
|---|---|---|
| Analyzed | Stable | |
| Spontaneous TK mutants | 10 | 10 |
| 0.25 | 10 | 3 |
| 0.5 | 10 | 0 |
| 0.75 | 10 | 1 |
| 1 | 10 | 1 |
| 1.25 | 10 | 1 |
| 1.5 | 10 | 0 |
| 4-NQOb | 10 | 10 |
aTFT resistance was evaluated by plating cells isolated from dAMPαS, TFT, or 4-NQO treated cultures in the presence or absence of TFT (4 µg/ml) as described in “Materials and Methods” section.
b4-NQO, 4-nitroquinoline-1-oxide (0.00033 mM).
Time Course of Colony Formation
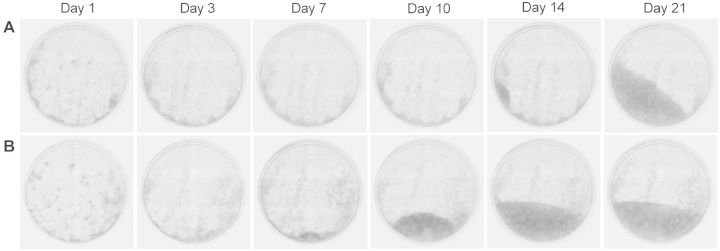
One explanation for the unstable TFT-resistant phenotype was that a fraction of parental TK6 cells were able to survive in the presence of TFT, and then formed colonies after the cytotoxicity of TFT is lost. If this was the case, we would expect delayed growth kinetics of dAMPαS-induced colonies and associated degradation of TFT in the culture medium. Accordingly, we first evaluated the time course of colony formation during the TFT-resistant selection process by monitoring the growth of colonies by IncuCyte ZOOM over a period of 3 weeks. As treatment with 1 mM of dAMPαS resulted in acceptable cell viability (RTG ≤ 20%) and induced a potent mutagenic response, this concentration was selected for further mechanistic studies. Representative phase contrast images (from two independent experiments) of TK6 cells treated with dAMPαS (1 mM) or 4-NQO are shown in Figure 1. Colonies induced by dAMPαS required approximately 14 days to start growing, whereas those induced by 4-NQO were visible within 7 days (Fig. 1). This data suggest distinct growth kinetics of dAMPαS-induced colonies compared with those induced by 4-NQO.
FIG. 1.
Kinetics of colony formation in TK6 cells during the phenotype selection in TK gene-mutation assay. TK6 cells were treated with 1 mM dAMPαS (A), or with 0.33 µM 4-NQO (B). Cells were then seeded in 96-well plates to evaluate the TFT-resistant phenotype and images of the whole well were captured up to 3 weeks by the IncuCyte ZOOM using ×4 objective.
The Influence of Extended Incubation on the Stability of the Selective Agent
The previous results showed that dAMPαS-induced colonies required longer incubation time to start growing. As these colonies were temporarily TFT-resistant, we anticipated that TFT was degraded during this prolonged incubation, thus allowing parental TK6 to resume growing. To examine whether TFT was still effective during the delayed growth of these colonies, we evaluated the stability/activity of TFT in culture medium. In this experiment, the effect of pre-incubation time on the efficacy of TFT as a selective agent for spontaneously occurring TK mutants was assessed in which the time of pre-incubation at 37°C was varied (1 week or 2 weeks). As shown in Table 3, there was a only a marginal difference in the TFT-resistant mutants frequency using either freshly prepared TFT or TFT pre-incubated for 1 week, with cells having a background of mutant fraction of approximately 7 × 10−6. As the pre-incubation time increased to 2 weeks, the efficiency of TFT to recover TFT-resistant mutants was drastically reduced resulting in approximately 28-fold increase in the background frequency of TFT-resistant mutants (p < .01). By evaluating the growth of TK6 cells resuspended in TFT preincubated in culture medium at 37°C, the half life of TFT is estimated to be 9 days (Supplementary Fig. 1). These results indicated that the efficacy of TFT to select spontaneous TK mutants was almost abolished within 2 weeks of incubation at 37°C.
TABLE 3.
Effect of Pre-incubation Time on TFT Efficacy to Select Spontaneous TK Mutants
| TFT pre-incubation timea | TFT resistance (× 10−6)b |
|---|---|
| 0 week | 7.2 ± 0.8 |
| 1 week | 7.3 ± 0.2 |
| 2 weeks | 190.5 ± 9.2** |
aTFT (final concentration 4 µg/ml) in RPMI 1640 with 20% heat-inactivated horse serum was incubated at 37°C for the indicated time. TK6 cells were then re-suspended in the TFT solution, and the TFT resistance was determined by plating cells as described in “Materials and Methods” section.
bValues represent the mean ± SD of two independent experiments.**p < .01 as compared with 0 week pre-incubation time.
When TK6 cells were re-fed with fresh TFT (final concentration was equal to 4 µg/ml) on day 7 and day 14, the mutant frequency induced by dAMPαS was dramatically reduced (Table 4). On the contrary, 4-NQO-induced colonies were not affected by re-feeding with TFT. Therefore, the unstable TFT-resistant mutants induced by dAMPαS can be eliminated by re-feeding with fresh TFT, as previously reported (Liber et al., 1985).
TABLE 4.
Effect of Re-feeding With Fresh TFT on dAMPαS-Induced Mutants
| TK mutant frequency (× 10−6)a | TK mutant frequency (× 10−6)a after re-feeding | |
|---|---|---|
| Control | 7.6 ± 2.2 | 8.2 ± 2.6 |
| dAMPαS (1 mM) | 335.4 ± 15.0** | 7.2 ± 1.7 |
| 4-NQOb | 71.4 ± 12.2* | 76.1 ± 17.5* |
aValues represent the mean ± SD of two independent experiments. *p < .05 and **p < .01, as compared with untreated control.
b4-NQO, 4-nitroquinoline-1-oxide (0.00033 mM).
We further investigated whether the activity of 6-TG, the selective agent used in the HPRT mutation assay, was maintained during mutant selection. As shown in Table 5, pre-incubation at 37°C failed to reduce the efficacy of 6-TG to select HPRT mutants even after 2 weeks of incubation. These results suggest that, unlike TFT, 6-TG has longer effective lifetime to select mutants.
TABLE 5.
Effect of Pre-incubation Time on 6-TG Efficacy to Select Spontaneous HPRT Mutants
| 6-TG pre-incubation timea | 6-TG resistance (× 10−6)b |
|---|---|
| 0 week | 1.4 ± 1.1 |
| 1 week | 1.3 ± 0.3 |
| 2 weeks | 1.8 ± 0.6 |
a6-TG (final concentration 5 µg/ml) in RPMI 1640 with 20% heat-inactivated horse serum was incubated at 37°C for the indicated time. TK6 cells were then re-suspended in the 6-TG solution, and the 6-TG resistance was determined by plating cells as described in “Materials and Methods” section.
bValues represent the mean ± SD of two independent experiments.
Cytotoxicity and Mutagenicity of PS Nucleotide Analog, dCMPαS
To investigate whether the increase in the unstable TFT-resistant mutants induced by dAMPαS is nucleobase-specific, TK6 cells were treated with a pyrimidine PS nucleotide analog, deoxycytidine monophosphorothioate (dCMPαS), and the genotoxicity was assessed at the TK and HPRT loci. As shown in Table 6, treatment with dCMPαS had minimal effect on cell viability (p value > .05). Interestingly, dCMPαS, at concentrations up to 1.5 mM, failed to induce TK or HPRT mutants above control (p value > .05).
TABLE 6.
Genotoxicity and Cytotoxicity of dCMPαS Nucleotide Analog in Human TK6 Cells
| dCMPαS (mM) | RTG (%)a | Mutant frequency (× 10−6)a |
|
|---|---|---|---|
| TK | HPRT | ||
| Control | 101.0 ± 2.8 | 7.5 ± 0.7 | 1.9 ± 0.1 |
| 0.25 | 113.0 ± 7.1 | 8.0 ± 1.4 | 2.5 ± 0.7 |
| 0.5 | 120.5 ± 6.3 | 4.5 ± 2.1 | 2.5 ± 0.7 |
| 0.75 | 99.5 ± 4.9 | 7.5 ± 0.7 | 2.0 ± 0 |
| 1 | 95.5 ± 4.9 | 7.5 ± 0.7 | 1.5 ± 0.7 |
| 1.25 | 98.1 ± 8.4 | 6.5 ± 2.1 | 4.0 ± 1.4 |
| 1.5 | 109.5 ± 9.2 | 9.5 ± 0.7 | 2.5 ± 0.8 |
| 4-NQOb | 19.0 ± 1.4 | 58.0 ± 10.0* | 17.5 ± 3.5* |
aValues represent the mean ± SD of two independent experiments. *p < .05 as compared with untreated control.
b4-NQO, 4-nitroquinoline-1-oxide (0.00033 mM).
Effect of dAMPαS on Cell Cycle in TK6 Cells
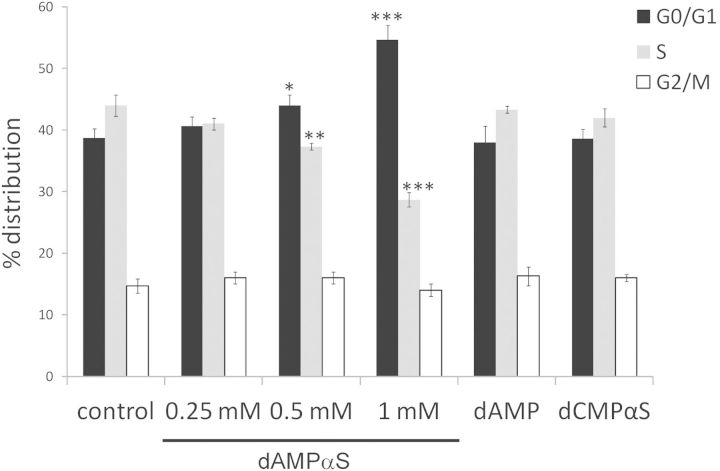
So far we have demonstrated that dAMPαS-induced colonies displayed delayed growth kinetics that appears to co-ordinate with the loss of TFT cytotoxicity. But how could parental TK6 cells escape the toxic effect of TFT during the first weeks of incubation? One simple explanation is that cell treatment with dAMPαS is associated with a reversible cell cycle arrest. This would allow the cells to survive in the presence of TFT and enable them to resume growing after the TFT is degraded. To test this hypothesis, the cell cycle status of control and treated TK6 cells were evaluated 48 h after exposure. Figure 2 shows that cells treated with 0.25 mM of dAMPαS exhibited a similar cell cycle progression to untreated cells (p > .05). In contrast, exposure of TK6 cells to higher doses of dAMPαS (0.5 and 1 mM) resulted in a dose-dependent cell cycle arrest at the G1 phase. The cell cycle profile of dAMP- or dCMPαS-treated cells was not significantly different from control (p value > .05).
FIG. 2.
Effect of dAMPαS on cell cycle in TK6 cells. TK6 cells were treated with different concentrations of dAMPαS, with 1 mM of dAMP or dCMPαS. Forty-eight hours after exposure, the cell cycle status was analyzed and compared with control untreated cells. Data represent the mean ± standard deviation (SD) of three independent experiments. *p < .05, **p < .01, and ***p < .001, as compared with control.
DISCUSSION
The aim of this study was to offer some insight into to the mechanism involved in the previously reported potent positive response induced by dAMPαS in the TK gene-mutation assay in TK6 cells (Reshat et al., 2012). Consistent with previous findings, we found that dAMPαS induces a dose-dependent increase in the apparent positive response at the TK locus (Reshat et al., 2012). Liber et al. (1985) using a similar experimental design as in this report, have showed that BrdUrd, a thymidine analog, induced a transient TFT-resistant phenotype that involved a nonmutagenic mechanism. In that work, the nonheritable resistance to TFT was attributed to the temporary loss of TK activity, possibly via BrdUrd incorporation into DNA. We were therefore concerned whether the increase in TK−/− mutants induced by dAMPαS might also result from a nonmutagenic mechanism. In fact, as shown previously with BrdUrd (Liber et al., 1985), we found that dAMPαS induced a transient, unstable TFT-resistant phenotype. We propose that it was likely that these unstable TFT-resistant colonies arose from a fraction of TK6 (tk+/−) cells that escaped the toxic effect of TFT, possibly via a transient growth arrest, and resumed growing after TFT was degraded. Consistent with this hypothesis, we found that the growth kinetics of dAMPαS-induced colonies was slower than 4-NQO-induced colonies, and appeared to co-ordinate with the loss of TFT efficacy in culture medium. The observation that dAMPαS induced a cell cycle arrest at G1 phase added weight to this hypothesis. When dAMP or dCMPαS, having minimal effect on the cell cycle, were tested, no increase in TK mutants was detected. In addition, the concentration at which dAMPαS induced the unstable TFT-resistant phenotype correlated with the induction of cell cycle arrest. Hence, taken together, these results indicate that the ability of TK6 cells to escape the toxic effect of TFT requires cell cycle arrest at the G1 phase. In this regard, the activity of TK, which phosphorylates TFT, has been shown to be cell cycle-dependent with minimal expression at G1 phase (Kauffman and Kelly, 1991). Therefore, the residual activity of TK during growth arrest at G1 phase may not be enough to convert sufficient TFT to its active monophosphate derivative. Following conversion to the monophosphate derivative, TFT can irreversibly inhibit the activity of thymidylate synthase, or be further converted to the triphosphate derivative and become incorporated into DNA resulting in replication errors or DNA strand breaks (Suzuki et al., 2011). Thus, treatment with dAMPαS may induce a fraction of wild-type TK6 cells to escape the toxic effect of TFT by cycle arrest at the G1 phase, minimizing the activity of TK responsible for TFT activation. The outgrowth of the temporarily arrested parental TK6 cells after the elimination of TFT activity would therefore explain the unstable TFT-resistant mutants induced by dAMPαS. We cannot exclude the possibility that other mechanisms are involved in the apparent positive response induced by dAMPαS. For example, hypermethylation has been suggested as a possible mechanism for BrdUrd-induced pseudomutational response at the TK locus in TK6 cells (Call and Thilly, 1991). However, in that work, BrdUrd had no effect on cell cycle progression, whereas dAMPαS induced cell cycle arrest in TK6 cells. Therefore, dAMPαS appears to differ from BrdUrd in its TK pseudomutational mechanism in TK6 cells.
Furthermore, we did not observe an increase in the “unstable” mutants at a different commonly used gene mutation marker gene, HPRT. We found that 6-TG, which retained its cytotoxic effect for longer time than TFT, prevented the re-growth of “leaky” parental TK6 cells, regardless of the cell cycle arrest, and thereby no increase in mutant frequency was observed at the HPRT locus. Consequently, if there was a fraction of parental growth arrested TK6 cells, it is unlikely that these non-mutants would skip the toxic effects of 6-TG. As recovery of the growth arrested cells required elimination of the selective agent from the culture medium, we concluded that the cell cycle arrest induced by dAMPαS was itself not sufficient to produce the unstable phenotype at HPRT locus as observed at the TK locus in TK6 cells.
It should be noted that the observed unstable TFT-resistant phenotype described in this report applies to the TK locus in TK6 cells and is not applicable to other cells lines that are heterozygous for the TK locus. For example, the mouse lymphoma assay (MLA), which is widely used as part of a standard in vitro battery of genotoxicity tests, also utilizes the TK locus to detect mutagens and clastogens in L5178Y tk+/− cells (Oberly et al., 1984). However, as MLA TK mutants are usually scored after 10 days of incubation, during which the cytotoxicity of TFT is maintained, the unstable TFT resistance phenotype would be eliminated. In addition, two phenotypic classes of TK mutants are usually generated from the TK6 assay including NG and SG mutants. Although an increase in SG mutants is usually associated with large intergenic mutations involving growth regulatory genes near the TK locus resulting in extended doubling time (> 21 h), NG mutants occur as a result of intragenic mutations (point mutations, small deletions, or insertions) and have similar doubling time (13–17 h) to the parental cells (Honma et al., 1997; Liber et al., 1987). Although colonies recovered from exposure to dAMPαS and after 3 weeks of incubation are not mutants, certain chemicals (clastogenic) are well known to induce SG TK mutants and actually represent true TK6 mutants (Liber and Denault, 1991; Zhan et al., 2004). Consequently, it would be incorrect to assume that any late-appearing mutants to be a false positive response but it is necessary to re-feed the cells with TFT to restore its cytotoxic effect and incubate cells for additional 7–10 days (Hakulinen et al., 2011; Liber and Denault, 1991; Liber et al., 1985).
Whereas our experiments indicated a cytotoxic effect of dAMPαS, Koziolkiewicz et al. (2001) have found that PS mononucleotides, regardless of the type of nucleobase, stimulated cell growth. Nonetheless, they showed that the difference in the cytotoxicity of mononucleotides was dependent on the different activity of the extracellular ecto-5′-nucleotidase in each cell line. This might, in part, explain the contrasting results observed with dAMPαS in TK6 cells. To our knowledge there are no data available regarding the G1 phase arrest mediated by PS nucleotides. However, there are several reports regarding adverse metabolic effects of the naturally occurring nucleoside, adenosine (Bynum, 1980; Fishman et al., 2000). Ishii and Green (1973) showed that treatment of human lymphoblast with adenosine, resulted in a cytostatic effect, possibly via inhibition of de novo pyrimidine synthesis. Whether such a mechanism could be applicable to dAMPαS remains to be determined. However, despite the structural similarity to dAMPαS, the unmodified nucleotide (dAMP) did not alter the cell cycle profile of TK6 cells, nor induced a mutagenic effect at either TK or HPRT loci in TK6 cells. This implies that a more complicated mechanism is responsible for dAMPαS-mediated growth arrest. This also suggests that treatment with a purine nucleotide per se is not sufficient to induce the adverse effects on cell growth. It is not unlikely for PS nucleotides to participate in enzymatic reactions (as inhibitors or activators) involved in the metabolism of endogenous nucleotides (Koziolkiewicz et al., 2001; Murray and Atkinson, 1968). However, any consequences resulting from interference with cellular metabolic activity might be simply augmented by the enhanced metabolic stability of these analogs (Cusack et al., 1983). Nonetheless, when we evaluated the genotoxic effects of a pyrimidine analog, dCMPαS in the TK and HPRT gene mutation assays in TK6 cells, we could not detect a positive response. Hence, the effect on cell growth mediated by PS nucleotides appeared to be nucleobase-specific and cannot be attributed solely to their enhanced nuclease stability. A similar observation was reported regarding the cytotoxicity of unmodified deoxynucleotide-5′-phosphates (dNMPs) which was found to be dependent on their nature and concentration (Vaerman et al., 1997). For example, deoxycytidine-5′-phosphate (dCMP) did not show any toxicity in hematologic cells as compared with other dNMPs used at the same concentration (Vaerman et al., 1997). It would be of interest to examine the remaining nucleobases in PS nucleotide analogs.
We have not carried out experiments to demonstrate cellular uptake of the modified nucleotide. Due to the negative charge on their phosphates, nucleotides in general are not highly cell permeable which may underestimate the metabolic/mutagenic effect reported here. Despite this limitation, the cytotoxic and cell cycle effect observed with dAMPαS would, at least partially, indicate a degree of cellular exposure. It would be difficult to expect such a positive effect on cell growth from a poorly internalized nucleotide (unless acting on an extracellular target). In addition, treating TK6 cells with the unmodified nucleoside, deoxyadenosine, resulted in a modest increase (1.8-fold) in TK mutants above control (R. Reshat, personal communication). Therefore, even if we assume conversion of dAMPαS to its corresponding nucleoside, or improved cellular uptake of the nucleoside, the increase in mutant frequency mediated by deoxyadenosine could not be explain the dramatic increase in the TK mutant frequency observed with dAMPαS in TK6 cells.
Because of their nuclease stability, PS-ONDs are wildly used in antisense applications. Systemically administered PS-ONDs are rapidly cleared from the blood and distributed to tissues (mainly liver and kidney) where they are metabolized by exonucleases into ONDs shortened from the 3’-end by one, two, or three nucleotides (Henry et al., 2002). The concern that these released nucleotides could perturb the endogenous nucleotide pools, or become incorporated into newly synthesized DNA with potential mutagenic consequence seems overstated. Even if these nucleotide analogs become incorporated into natural DNA, the modified backbones would maintain the phosphodiester backbone and the natural Watson–Crick base pairing, and thus still serve as a genetic template (Thaler et al., 1996). Furthermore, considering their chemical structure, PS nucleotides are unlikely to directly interact and/or damage chromosome/DNA. Our findings from the TK and HPRT gene-mutation assay and the micronucleus test in TK6 cells, suggest that PS nucleotides, or at least dAMPαS, are unlikely to induce a mutagenic event. In support of our results, using the standard battery of in vitro and in vivo genotoxicity tests including bacterial reverse mutation test, a chromosomal aberration test, mammalian cell gene-mutation assay in L5178Y cells and bone marrow mouse micronucleus test, Henry et al. (2002) found no evidence of genotoxicity of a 21-mer PS-OND. Furthermore, the majority of mononucleotides generated from the degradation of ONDs are excreted in the urine or catabolized (Agrawal et al., 1991).
In conclusion, we have demonstrated that dAMPαS induces a transient TFT-resistant phenotype that did not involve a mutational mechanism. We recommend that any interpretation of TK gene-mutation data in TK6 cells, especially with SG mutants, should take into account the possibility of temporarily TFT-resistant parental cells escaping the TFT selection. Failure to do so could potentially lead to unexpected false positive responses in the TK gene-mutation assay in TK6 cells, in particular when evaluating the genotoxicity of nucleotide analogs.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Agrawal S., Temsamani J., Tang J. Y. (1991). Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc. Natl Acad. Sci. U.S.A. 88, 7595–7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D., Richardson C. R., Davies P. J. (1981). The genotoxic potential of bases and nucleosides. Mutat. Res. 91, 265–272. [DOI] [PubMed] [Google Scholar]
- Brox L., Ng A., Pollock E., Belch A. (1984). DNA strand breaks induced in human T-lymphocytes by the combination of deoxyadenosine and deoxycoformycin. Cancer Res. 44, 934–937. [PubMed] [Google Scholar]
- Bynum J. W. (1980). Characterization of adenosine-induced cytostasis in melanoma cells. Cancer Res. 40, 2147–2152. [PubMed] [Google Scholar]
- Call K. M., Thilly W. G. (1991). 5-Azacytidine inhibits the induction of transient TK-deficient cells by 5-bromodeoxyuridine. A novel hypothesis for the facilitation of hypermethylation by 5-bromodeoxyuridine. Mutat. Res. 248, 101–114. [DOI] [PubMed] [Google Scholar]
- Clements J. (2000). The mouse lymphoma assay. Mutat. Res. 455, 97–110. [DOI] [PubMed] [Google Scholar]
- Clive D., Flamm W. G., Machesko M. R., Bernheim N. J. (1972). A mutational assay system using the thymidine kinase locus in mouse lymphoma cells. Mutat. Res. 16, 77–87. [DOI] [PubMed] [Google Scholar]
- Cusack N. J., Pearson J. D., Gordon J. L. (1983). Stereoselectivity of ectonucleotidases on vascular endothelial cells. Biochem. J. 214, 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleavey G. F., Damha M. J. (2012). Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 19, 937–954. [DOI] [PubMed] [Google Scholar]
- Eckstein F. (2000). Phosphorothioate oligodeoxynucleotides: What is their origin and what is unique about them? Antisense Nucleic Acid Drug Dev. 10, 117–121. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. (1979). Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: Spontaneous mutation by misincorporation. Proc. Natl Acad. Sci. U.S.A. 76,4946–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P., Bar-Yehuda S., Ohana G., Pathak S., Wasserman L., Barer F., Multani A. S. (2000). Adenosine acts as an inhibitor of lymphoma cell growth: A major role for the A3 adenosine receptor. Eur. J. Cancer 36, 1452–1458. [DOI] [PubMed] [Google Scholar]
- Geary R. S., Leeds J. M., Fitchett J., Burckin T., Truong L., Spainhour C., Creek M., Levin A. A. (1997). Pharmacokinetics and metabolism in mice of a phosphorothioate oligonucleotide antisense inhibitor of C-raf-1 kinase expression. Drug Metab. Dispos. 25, 1272–1281. [PubMed] [Google Scholar]
- Graham M. J., Lee R. G., Bell T. A., 3rd, Fu W., Mullick A. E., Alexander V. J., Singleton W., Viney N., Geary R., Su J., et al. (2013). Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ. Res. 112, 1479–1490. [DOI] [PubMed] [Google Scholar]
- Hakulinen P., Yamamoto A., Koyama N., Kumita W., Yasui M., Honma M. (2011). Induction of TK mutations in human lymphoblastoid TK6 cells by the rat carcinogen 3-chloro-4 -(dichloromethyl)-5-hydroxy-2(5 H)-furanone (MX). Mutat. Res. 725, 43–49. [DOI] [PubMed] [Google Scholar]
- Henry S. P., Monteith D. K., Matson J. E., Mathison B. H., Loveday K. S., Winegar R. A., Matson J. E., Lee P. S., Riccio E. S., Bakke J. P., et al. (2002). Assessment of the genotoxic potential of ISIS 2302: A phosphorothioate oligodeoxynucleotide. Mutagenesis 17, 201–209. [DOI] [PubMed] [Google Scholar]
- Honma M. (2005). Generation of loss of heterozygosity and its dependency on p53 status in human lymphoblastoid cells. Environ. Mol. Mutagen. 45, 162–176. [DOI] [PubMed] [Google Scholar]
- Honma M., Hayashi M., Sofuni T. (1997). Cytotoxic and mutagenic responses to X-rays and chemical mutagens in normal and p53-mutated human lymphoblastoid cells. Mutat. Res. 374, 89–98. [DOI] [PubMed] [Google Scholar]
- Ishii K., Green H. (1973). Lethality of adenosine for cultured mammalian cells by interference with pyrimidine biosynthesis. J. Cell Sci. 13, 429–439. [DOI] [PubMed] [Google Scholar]
- Kauffman M. G., Kelly T. J. (1991). Cell cycle regulation of thymidine kinase: Residues near the carboxyl terminus are essential for the specific degradation of the enzyme at mitosis. Mol. Cell Biol. 11, 2538–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziolkiewicz M., Gendaszewska E., Maszewska M., Stein C. A., Stec W. J. (2001). The mononucleotide-dependent, nonantisense mechanism of action of phosphodiester and phosphorothioate oligonucleotides depends upon the activity of an ecto-5'-nucleotidase. Blood 98, 995–1002. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Virolainen M., Defendi V. (1968). Human lymphoblastoid lines from lymph node and spleen. Cancer 22, 517–524. [DOI] [PubMed] [Google Scholar]
- Liber H. L., Denault C. M. (1991). Mutagenicity of 2-amino-N6-hydroxyadenine to TK6 human lymphoblast cells. Mutat. Res. 253, 91–95. [DOI] [PubMed] [Google Scholar]
- Liber H. L., Call K. M., Little J. B. (1987). Molecular and biochemical analyses of spontaneous and X-ray-induced mutants in human lymphoblastoid cells. Mutat. Res. 178, 143–153. [DOI] [PubMed] [Google Scholar]
- Liber H. L., Call K. M., Mascioli D. A., Thilly W. G. (1985). Mutational and pseudomutational effects of 5-bromodeoxyuridine in human lymphoblasts. Mutat. Res. 151, 95–108. [DOI] [PubMed] [Google Scholar]
- Liber H. L., Yandell D. W., Little J. B. (1989). A comparison of mutation induction at the tk and hprt loci in human lymphoblastoid cells; quantitative differences are due to an additional class of mutations at the autosomal tk locus. Mutat. Res. 216, 9–17. [DOI] [PubMed] [Google Scholar]
- Mattano S. S., Palella T. D., Mitchell B. S. (1990). Mutations induced at the hypoxanthine-guanine phosphoribosyltransferase locus of human T-lymphoblasts by perturbations of purine deoxyribonucleoside triphosphate pools. Cancer Res. 50, 4566–4571. [PubMed] [Google Scholar]
- Molloy J., Doherty A. T., Chocian K., Hayes J., O’Donovan M. (2010). Karyotypic analysis and stability with time in culture of TK6 and L5178Y cell lines. Mutagenesis 25, 645. [Google Scholar]
- Murray A. W., Atkinson M. R. (1968). Adenosine 5'-phosphorothioate. A nucleotide analog that is a substrate, competitive inhibitor, or regulator of some enzymes that interact with adenosine 5'-phosphate. Biochemistry 7, 4023–4029. [DOI] [PubMed] [Google Scholar]
- Oberly T. J., Bewsey B. J., Probst G. S. (1984). An evaluation of the L5178Y TK+/- mouse lymphoma forward mutation assay using 42 chemicals. Mutat. Res. 125, 291–306. [DOI] [PubMed] [Google Scholar]
- Reshat R., Priestley C. C., Gooderham N. J. (2012). Mutagenesis by an antisense oligonucleotide and its degradation product. Toxicol. Sci. 130, 319–327. [DOI] [PubMed] [Google Scholar]
- Ryan T. J., Boddington M. M., Spriggs A. I. (1965). Chromosomal abnormalities produced by folic acid antagonists. Br. J. Dermatol. 77, 541–55. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Liber H. L., Penman B. W., Thilly W. G. (1978). Isolation of a human lymphoblastoid line heterozygous at the thymidine kinase locus: Possibility for a rapid human cell mutation assay. Biochem. Biophys. Res. Commun. 84, 411–416. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Nakagawa F., Nukatsuka M., Fukushima M. (2011). Trifluorothymidine exhibits potent antitumor activity via the induction of DNA double-strand breaks. Exp. Ther. Med. 2, 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler D. S., Liu S., Tombline G. (1996). Extending the chemistry that supports genetic information transfer in vivo: Phosphorothioate DNA, phosphorothioate RNA, 2'-O-methyl RNA, and methylphosphonate DNA. Proc. Natl Acad. Sci. U.S.A. 93, 1352–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. S., Cromwell W. C., Ali S., Chin W., Flaim J. D., Davidson M. (2013). Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 62, 2178–2184. [DOI] [PubMed] [Google Scholar]
- Vaerman J. L., Moureau P., Deldime F., Lewalle P., Lammineur C., Morschhauser F., Martiat P. (1997). Antisense oligodeoxyribonucleotides suppress hematologic cell growth through stepwise release of deoxyribonucleotides. Blood 90, 331–339. [PubMed] [Google Scholar]
- Zhan L., Sakamoto H., Sakuraba M., Wu D. S., Zhang L. S., Suzuki T., Hayashi M., Honma M. (2004). Genotoxicity of microcystin-LR in human lymphoblastoid TK6 cells. Mutat. Res. 557, 1–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.