Abstract
In vitro exposures to air pollutants could, in theory, facilitate a rapid and detailed assessment of molecular mechanisms of toxicity. However, it is difficult to ensure that the dose of a gaseous pollutant to cells in tissue culture is similar to that of the same cells during in vivo exposure of a living person. The goal of the present study was to compare the dose and effect of O3 in airway cells of humans exposed in vivo to that of human cells exposed in vitro. Ten subjects breathed labeled O3 (18O3, 0.3 ppm, 2 h) while exercising intermittently. Bronchial brush biopsies and lung lavage fluids were collected 1 h post exposure for in vivo data whereas in vitro data were obtained from primary cultures of human bronchial epithelial cells exposed to 0.25–1.0 ppm 18O3 for 2 h. The O3 dose to the cells was defined as the level of 18O incorporation and the O3 effect as the fold increase in expression of inflammatory marker genes (IL-8 and COX-2). Dose and effect in cells removed from in vivo exposed subjects were lower than in cells exposed to the same 18O3 concentration in vitro suggesting upper airway O3 scrubbing in vivo. Cells collected by lavage as well as previous studies in monkeys show that cells deeper in the lung receive a higher O3 dose than cells in the bronchus. We conclude that the methods used herein show promise for replicating and comparing the in vivo dose and effect of O3 in an in vitro system.
Keywords: ozone, in vivo versus in vitro dose, extrapolation, epithelial cells, bronchoalveolar lavage
Recent proposals for identifying exposure hazards to toxic agents involve the identification of “toxicity pathways” in primary cultures of human cells. The possibility of extrapolating toxicity observed in vitro to human risk in vivo is attractive because it might allow a deeper understanding of the molecular basis of injury through use of “omics” techniques as well as the possibility of high throughput screening. Air pollutant gases and aerosols present a unique challenge to in vitro work because of difficulty replicating the dose and exposure conditions found in vivo. Few studies have attempted to re-create in vitro the dose and effect of an air pollutant observed in vivo. (National Research Council, 2007).
The goal of the present study was to contribute a proof-of-concept for direct extrapolation of air pollutant toxicity from in vitro cell cultures to in vivo human bronchial epithelial cells. Epithelial cells were chosen because they secrete mediators which initiate inflammatory events that are believed to be important in human risk assessments. They also can be expanded in air-liquid interface cultures and used in high throughput assays.
Ozone (O3) was chosen as a prototype pollutant because it is one of the best-studied and ubiquitous of pure gaseous inhalants (U. S. E. P. A., 2013).
We showed previously that exposure to heavy oxygen-labeled ozone (18O3) results in incorporation of 18O into cells and extracellular material obtained from bronchoalveolar lavage fluid (BALF) of humans and animals (Hatch et al., 1994, 2013; Plopper et al., 1998) creating a convenient micro-measurement of O3 dose that is related to O3 toxicity. We have also demonstrated that epithelial cells release mediators following O3 exposure that are early markers of inflammation and that appear to have health consequences in the human population (Devlin et al., 1996; Devlin et al., 2012). The generation of these mediators can be quantified by gene expression assays. We here compare the 18O3 dose and gene expression of two inflammatory mediators in cells obtained from human subjects exposed in vivo with the same measurements made in human primary cells expanded in culture and exposed in an air-liquid interface system in vitro.
MATERIALS AND METHODS
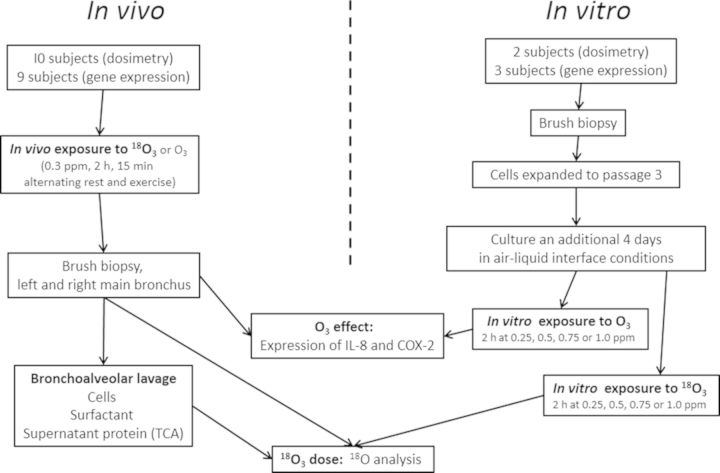
A flow chart depicting the study design is shown in Figure 1. In vivo exposures to 18O3 (for dose measurement) and unlabeled O3 for gene expression measurement were compared with in vitro exposures of the same length (2 h) that measured the same dose and effect endpoints. In vitro exposures to 18O3 were performed on cells expanded from two subjects, and gene expression changes were evaluated in cells expanded from three subjects. Both the dose and effect in vitro studies employed the same range of 18O3 or unlabeled O3 concentrations. Cells were harvested at the termination of exposure in both the in vivo and in vitro studies.
FIG. 1.
Flow chart of the experimental design. The study involved both an in vivo and an in vitro exposure to 18O3 of human epithelial cells. Bronchoalveolar lavage cells and constituents were also measured for comparison with the epithelial cells. Biological effects of O3 consisted of measurements of IL-8 and COX-2 gene expression in cells harvested after exposure to unlabeled O3. Cells were sampled 1 h after in vivo exposures and immediately following the in vitro exposures.
In Vivo 18O3 Exposures of Human Subjects
For the dosimetry of ten subjects, eight (seven Caucasian and one Hispanic) were male and two (one Caucasian and one Hispanic) were female, and the following data apply: age 26 ± 1 years (19–33 years), body weight 80.8 ± 2.8 kg, height 176.9 ± 3.0 cm, and resting forced vital capacity (FVC) 5.55 ± 0.27 l. For the biological effects study of nine subjects, all were males (one Black, one Hispanic and seven Caucasian), with the following characteristics: age 23.7 ± 0.9 (20–29 years), body weight 86.1 ± 2.6 kg, height 182.0 ± 2.0 cm, and resting FVC 5.88 ± 0.2 l. The study protocol was approved by the University of North Carolina Committee on the Rights of Human Subjects (Institutional Review Board) as well as the U.S. Environmental Protection Agency. All individuals provided written informed consent and were recruited under a contract to the Westat Corp. (Rockville, MD). All subjects underwent a physical examination, with a complete blood count and differential, serum electrolytes, glucose, and liver enzymes. Female subjects had a negative urine pregnancy test. Volunteers were excluded from the study if they had a smoking history within 2 years of the study, or were not able to refrain from taking over-the-counter anti-inflammatory agents, vitamins, or antioxidants for at least a week prior to exposure. Participants who reported a recent respiratory tract infection (or who had symptoms) were not studied for a period of at least 6 weeks after symptoms had disappeared.
Each volunteer was exposed on a single occasion for 2 h to 18O3. Subjects were seated on a bicycle ergometer with their forehead touching a pad on the top of a 6 inch diameter cone that blew 18O3 downward across the face at a flow rate of 500 l/min. The ozone concentration delivered did not deviate from the target concentration by more than ±5%. A sampling tube (with a flow rate of 2–2.5 l/min) similar to a head-mounted microphone collected breathing air 1–2 inches in front of the face of the subject. 18O3 concentrations were thus monitored in the breathing zone every 10 s and stored as 2 min averages. Mean concentrations in the breathing zone ranged from 0.21 to 0.33 ppm and were 30–50% lower than in the incoming airstream.
Throughout the exposure, volunteers underwent moderate intermittent exercise (15 min intervals) on the bicycle ergometer. The level of exercise was targeted to a minute ventilation (Ve) of 25 l/min/m2 body surface area (∼50 l/min/subject). Prior to the exposure day, all subjects participated in a training session to establish the workload that would be required to achieve this Ve and the same work load was targeted on the ergometer. Subjects breathed naturally by mouth or nose depending on the subjects' comfort level. On the study day, all subjects performed a pulmonary function test prior to the exposure using a Sensormedics Vmax 220 instrument and software (Sensormedics Corp., Yorba Linda, CA) according to American Thoracic Society guidelines. Spirometry was also conducted on all subjects immediately following the exposure. Decrements in FEV1 were <20% for all subjects post- versus pre-exposure. All volunteers had forced expiratory volume in 1 s (FEV1) and FVC baseline values of at least 80% of that predicted for their height, age, and gender. Subjects were outfitted with a telemetry unit for the duration of the 18O3 exposure and bronchoscopy for continuous monitoring of heart rate and rhythm as well as finger oxidimetry for O2 saturation. Each subject was exposed twice, once to air and once to O3, separated by at least 2 weeks.
In vivo 18O3 and O3 generation
The 18O3 and unlabeled O3 exposures were conducted identically in a chamber (dimensions of 1.22 m wide × 2.44 m long × 1.83 m high) at the U.S. Environmental Protection Agency Human Studies Facility on the campus of the University of North Carolina, Chapel Hill, NC. Air from an oil-less compressor was passed through an air purifier (ZEKS Nomonox model CDP480CD00, West Chester, PA). It was then humidified by passage over heated deionized water. 18O3 was generated by passing a 1–2% stream of 18O2 (Isotec, Inc., Miamisburg, OH; 99 atom %) in argon through a silent electric discharge (model CMGK-F 0.5 O3 generator; Innovatec Gerätetechnik GmbH, Rheinbach, Germany). The 18O3 concentration was controlled by altering the flow of 18O2 into a constant flow of argon that passed through the generator. Chamber relative humidity and temperature were measured once a second and stored as 2 min averages that yielded an overall mean and SD of 39.1 ± 0.2% and 22.5 ± 0.1°C, respectively.
Exposures to unlabeled O3 were performed by metering pure O2, along with dilution air, into a silent arc O3 generator. O3 concentration was controlled by varying the power to the generator, but otherwise the exposures were done identically to the 18O3 exposures.
Bronchoscopic sample collection
A bronchoscopy was conducted ∼45 min following the completion of the 2 h exposure to 18O3 as described previously (Ghio et al., 1998) modified as follows. Immediately prior to the procedure, subjects were given intravenous fluids through a saline lock catheter placed in an antecubital vein to ensure they were hydrated. Primary bronchial epithelial cells were obtained by brush biopsy of both the right and left mainstem bronchus. Each bronchus was brushed six times in and out with a ∼5 cm excursion, and then repeated with a second brush. The two cytology brushes from each side were kept separate and the epithelial cells dislodged by agitation and brief vortexing in 0.1 ml of cold Dulbecco's phosphate buffered saline. The dislodged cells were pelleted by centrifugation at 400 × g at 4°C. For 18O measurements, the supernatant was removed and the remaining cell pellet was lysed by pipetting up and down in 0.1 ml of 0.05% SDS. This solution was then diluted by adding an additional 0.1 ml of water.
Bronchoalveolar lavage was conducted after the collection of the brush biopsies. Three 50 ml aliquots of sterile saline were sequentially injected into the right middle lung lobe. After each aliquot of saline was instilled it was gently aspirated back into the same syringe. The unfiltered lavage fluid was placed on ice in 50 ml conical tubes. Mean percent recovery volumes (range) of BALF from each lavage were as follows: wash no. 1; 29.4 ± 7.0% (14–38%), wash no. 2; 67.4 ± 9.2% (52–84%), wash no. 3; 77.8 ± 11.4% (58–92%). Samples were immediately centrifuged at 1000 × g for 10 min at 4°C to pellet the cell fraction. BALF supernatants from all three aliquots were pooled and stored at −80°C for subsequent analysis of 18O/16O content. Cell pellets from each aliquot were individually lysed by the addition of 0.1 ml of 0.05% SDS and the resulting cell lysates were dispersed by pipetting up and down and then diluted with an equal volume of water.
Primary Human Epithelial Cell Cultures and 18O3 Exposures
Cell collection and culture
Two dose-response in vitro18O3 exposure experiments were performed to measure 18O and three to measure mRNAs' coding for pro-inflammatory mediators. Each concentration had three technical replicates that were averaged. Each experiment used cells originating from bronchial brush biopsies from a separate volunteer that participated in the in vivo exposure experiments. Three wells (technical replicas) were exposed. Cells were expanded to passage three in bronchial epithelial growth medium (Lonza, San Diego, CA). This media contained a background level of 0.83 mg/ml protein, 0.16nM retinoic acid, and no ascorbic acid. Cells were plated on Costar Trans-Clear filter supports with a 0.2µm pore size (Costar Corp., Cambridge, MA) and inserted into 12-well tissue culture plates (22.1 mm diameter wells; Corning Inc., Wilkes-Barre, PA). Each filter support was pre-coated by addition of 0.2 ml of 40 ug/ml collagen solution. Culture media under the interface (1 ml volume) was enriched to 0.1uM retinoic acid after the cells reached 100% confluence. Air-liquid interface culture conditions were initiated 24–48 h after the addition of retinoic acid by removing the apical medium. The cells were maintained at air-liquid interface conditions by replacing the basolateral medium (containing 0.1uM retinoic acid) every 48 h for 4 days. The last medium exchange was always done immediately prior to the 18O3 exposure. At this stage of passage, cells were not yet producing a measurable mucus layer on their surface.
In vitro 18O3 exposure of epithelial cells
Cells were exposed to four 18O3 concentrations and an air control (0.0, 0.25, 0.50, 0.75, or 1.0 ppm 18O3) for 2 h on the same day. No lid was present and atmospheres consisted of filtered air or 18O3 in 5% CO2 and maintained at 37°C and 88% relative humidity. Previous studies by us and others have shown that these concentrations do not cause more than minimal (5% or less) decreases in cell viability as measured with lactate dehydrogenase release assay. The exposure chambers were 30 × 30 × 40 cm stainless steel boxes with airflow passing from top to bottom through porous shelves. They were housed inside Nuaire Incubators (Plymouth, MN) that kept the temperature constant and provided the necessary CO2 analyzer. Air flowing through the chambers originated from an oil-less compressor that was passed through a purifier (Aacdo Medical Inc., Cleves, OH) and a humidifier (Pure Steam, Chaska, MN) and pumped from the chamber using a Fox Venture Eductor (Dover, NJ). 18O3 was generated similar to the in vivo exposures except that the 18O3 generator was taken from a Bendix NO/NOx analyzer (Lewisburg, WV). Chamber concentrations of 18O3 were monitored using TECO 49i O3 analyzers (Thermo Science, Franklin, MA) and controlled by altering the flow rate of an argon/18O2 mixture passing through the O3 generator.
In both in vivo and in vitro exposures, there was a small excess (100–200 ppm) of 18O2 resulting from incomplete conversion of 18O2 to 18O3 (conversion efficiency is 1–2%). We have shown that 18O2 exposure at 21% results in 18O incorporation, but that 18O3 incorporation is 142,000- to 210,000-fold higher on a per ppm basis (Supplementary table 1). This difference in incorporation appears to be the result of higher chemical reactivity of the 18O3 and is evident even though the incorporation of 18O2 into cells also includes uptake resulting from normal metabolic processes.
Tissue and BALF Fraction Preparation for 18O Analysis
BALF cell pellets were resuspended in 0.2 ml of 0.025% SDS. BALF supernatants were combined and centrifuged for 1 h at 22,000 × g to obtain a white “surfactant pellet.” The resulting dried supernatant contained proteins and lipids mixed in a high NaCl matrix that could interfere with measurement of 18O. For this reason, we precipitated the protein by adding trichloroacetic acid (TCA) to a final concentration of 10% and centrifuged again (40,000 × g for 1 h at 4°C). The surfactant and TCA pellets were resuspended in 0.4 ml of the 0.025% SDS solution.
Immediately following the in vitro exposures, cells were analyzed for 18O content. This time point was chosen to minimize metabolism of the 18O signal by cells and to coincide with the same time point in which ozone-induced changes in mRNAs coding for pro-inflammatory proteins have been observed. Cells were lysed by the addition of 0.1 ml of 0.05% SDS to the apical surface. The cells were dislodged from the filter support using a pipette tip and the lysate transferred to a cryovial. The filter support was rinsed with an additional 0.1 ml of water and this was pooled with the original 0.1 ml of cell lysate for a final SDS concentration of 0.025% in the final sample. Epithelial cell SDS lysates from both the in vivo and in vitro18O3 exposures and BALF cells from the in vivo exposures were stored at −80°C, then lyophilized and stored with desiccant at 4°C.
Analysis of 18O in lyophilized samples has been described previously (Hatch et al., 1994; Santrock et al., 1989). Briefly, lyophilized samples weighing 0.5–1.5 mg were placed into silver cups (3 × 5 mm), crimped, and then placed into an elemental analyzer (Carlo Erba Instruments, Italy) where all oxygen in the samples was converted to CO under a stream of pure helium. The elemental analyzer quantified the CO which yielded a percentage (per dry weight) of total elemental oxygen in each sample. The helium/CO effluent of the elemental analyzer was captured and passed by continuous flow through a heated (110°C) column of granular I2O5 (to convert CO to CO2), then a cryogenic trap (−60°C to remove produced I2), and then bled by capillary into the vacuum of an isotope ratio mass spectrometer (SIRA 10, VG instruments) where the ratios between CO2 masses were determined. 18O3 exposed samples were compared with their natural abundance controls inserted into each run.
Quantitative RT-PCR for Detection of Gene Expression Changes
Gene expression changes of the inflammatory markers IL-8 and COX-2 in human airway epithelial cells were quantified using reverse transcription polymerase chain reaction (RT-PCR). Total RNA was isolated using an RNeasy Mini Kit according to manufacturer's instructions (Qiagen, Valencia, CA). RNA quantity was determined on a Nanodrop ND-1000 and RNA integrity was assessed using RNA6000 Nano chips on a Bioanalyzer 2100 (Agilent). Total RNA (100–200 ng) was reverse transcribed to generate cDNA using the High Capacity cDNA Reverse Transcription kit (Life Technologies Applied Biosystems, Foster City, CA). Quantitative fluorogenic amplification of cDNA was performed using the ABI StepOnePlusTM Real-Time PCR System (Applied Biosystems) with iTaq Universal Probes Supermix (Bio-Rad), primer/probe sets for the target gene of interest multiplexed with b-actin (ACTB) primer/probe sets as the normalizing housekeeping gene (20× VIC/MGB, Primer Limited, Applied Biosystems). Values from triplicate technical replicates for each sample were averaged and data analyzed using the DDCt method to obtain fold change values following ozone exposure with respect to air exposure values.
Data Analysis
The “incorporation of 18O” is defined here as the measure of O3 dose. A18O/16O ratio was derived from the relative CO2 masses obtained from the mass spectrometer as a unitless “delta value.” The enrichment in 18O in the 18O3 exposed samples was quantified and units changed to umoles of 18O/mole of total oxygen by subtracting the mean of the background natural abundance of 18O from all samples. This background, consisting of ∼0.2 atom % of all oxygen, was obtained from analysis of air-exposed cells in the case of in vitro data or from blood plasma samples of the subjects in the case of in vivo exposures. The denominator for the enrichment in 18O was then converted to per gram dry weight by use of the mean elemental percentage of oxygen (obtained as output from the elemental analyzer). This value was obtained from 10 subjects, each exposed to both clean air and ozone, and paired t-tests were used to determine statistical significance (p < 0.05). For in vitro exposure experiments, one-way ANOVA was used to calculate significance.
Analysis of gene expression performed on in vivo exposed samples involved paired t-test with each subject serving as his/her own control. Analysis of in vitro gene expression data was performed by ANOVA followed by Dunnett's test of individual means.
RESULTS
Methodology Findings
The cells obtained by brush biopsy were estimated to be greater than 95% epithelial cells. We originally explored trying to determine the percentage of different types of epithelial cells in the biopsies but they come off the brush in clumps making this impossible. The only cells that grow from these biopsies are the basal cells. Ciliated cells, mucus cells, clara cells, etc. are all terminally differentiated and do not divide in culture. Cell counts and differentials obtained from BALF are shown in Supplementary table 2. Percentage of polymorphonuclear leukocytes (PMNs) was increased by O3 exposure at both the 1 h and 24 h BALF collection times.
We were able to obtain sufficient sample from a single culture well of confluent epithelial cells, or from the brush biopsy from one main bronchus. BALF cells and surfactant pellets yielded larger samples than were obtained from TCA pellets of BALF. Our 18O analysis required 0.5–1.5 mg of dry weight with a total oxygen percentage >10%. The bronchial biopsies barely met these requirements and for this reason the gene expression changes could not have been measured in the same samples as the 18O dose. The detection limit for the 18O assay depends on (1) the quantity of elemental oxygen in the sample (accuracy improves with higher oxygen/sample) and (2) number of replicate samples (accuracy improves with higher number of samples analyzed due to both “memory” from the previous samples and increased statistical power with larger sample numbers). Dry weights of brush biopsy samples are shown in Supplementary table 3. The 18O values for the left and right bronchial brush biopsies were averaged because there was no correlation between the two measurements. The use of SDS for lysis and dispersion of samples prevented sticking of dry sample to the walls of the tubes that facilitated weighing the samples into silver sampling cups after lyophilization. The contribution of SDS to the dry weight of the samples was ∼10% for the BALF cell and surfactant fractions, and 5% for the other samples.
Elemental Oxygen Percentages
Oxygen percentages that were used in converting the units of 18O dose as described above were as follows (mean ± SE): in vitro epithelial cells expt. 1, 17.0 ± 0.7; in vitro epithelial cells expt. 2, 17.2 ± 1.2; in vivo bronchial brush biopsies, 12.7 ± 0.9; BALF cells, 16.2 ± 1.1; BALF supernatant TCA pellet, 11.9 ± 0.8; BALF surfactant pellet, 18.0 ± 0.2; plasma 1, 23.8 ± 1.7; Plasma 2, 21.4 ± 1.1.
Comparison of In Vitro and In Vivo 18O3 Incorporation
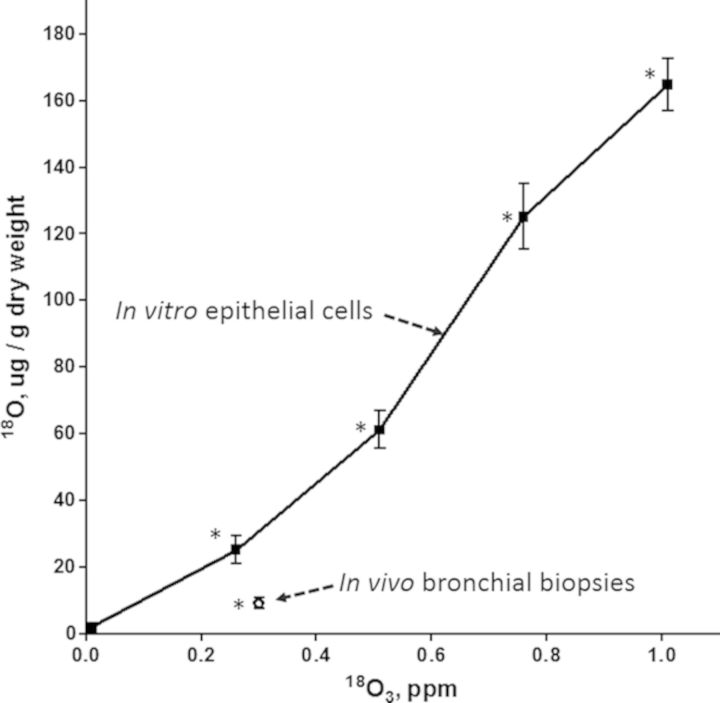
18O3 exposure resulted in significant incorporation of 18O following the in vivo study and each 18O3 concentration in the in vitro study (Fig. 2). The mean ± SE 18O values of bronchial brush biopsies taken after in vivo18O3 exposure are plotted along with the concentration response of 18O data from the in vitro exposure. In vivo exposed cells showed lower 18O incorporation than in vitro exposed cells at the same 18O3 concentration. For example, the 18O incorporation in vivo was 64% lower than that seen at the same 18O3 level (0.3 ppm) on the dose response curve of the in vitro exposed cells. We were unable with the present sample size to observe any relationships between 18O incorporation and subject age, sex, or race. Variability observed in the in vitro data could have been the result of (1) differences between the subjects from which cells were obtained, (2) variation in the two 18O analysis runs, or (3) between the replicate culture wells exposed at each 18O3 concentration. The variability in natural abundance of 18O (zero ppm 18O3) was small compared with variability among 18O3 exposed cells.
FIG. 2.
In vitro and in vivo dose comparison: 18O incorporation into bronchial brush biopsies collected from human subjects exposed for 2 h to 0.3 ppm 18O3 while exercising intermittently or human primary epithelial cells exposed in vitro to a range of 18O3 concentrations (mean ± SE). Standard error bars are within the data point for the in vivo data (N = 10 human subjects for in vivo data and 5 culture wells derived from two replicate in vitro exposures to each 18O3 concentration for in vitro data). Each mean shown is significantly elevated (* denotes p < 0.05) relative to natural background 18O (zero 18O3).
Comparison of In Vitro and In Vivo O3 Biological Effects
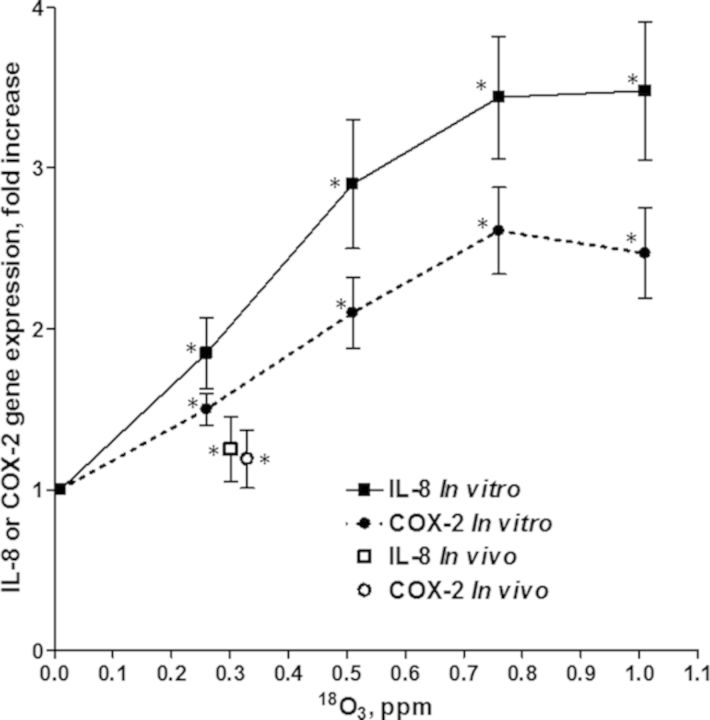
Figure 3 shows the fold increases (±SE) in gene expression of IL-8 and COX-2 in bronchial brush biopsies from in vivo O3 exposures and primary epithelial cell cultures exposed in vitro to the same range of O3 concentrations as was used for the 18O3 study. Similar to the 18O data, the in vivo gene expression was lower than that observed following in vitro exposure. The IL-8 increase in expression in the in vivo exposed cells fell about 30% below that observed in vitro and the in vivo COX-2 response fell about 17% below the response observed in vitro. The in vitro expression of IL-8 was higher than the expression of COX-2 but both genes were induced in a concentration-related manner that leveled off at 0.75 and 1.0 ppm O3. Although epithelial cells obtained from subjects exposed in vivo showed elevations in gene expression smaller than were observed at the lowest dose (0.25 ppm) used in the in vitro study, the increases in both genes were significant by paired t-test of the nine subjects studied.
FIG. 3.
In vitro and in vivo biological effects comparison of O3 exposures under conditions similar to the dose comparison in Figure 2. Shown are the fold increases in expression of IL-8 or COX-2 genes (means ± SE) in bronchial brush biopsies obtained from nine human subjects exposed in vivo to O3 compared with their response following exposure to clean air. Also shown are fold increases of primary epithelial cells exposed in vitro to each O3 concentration (means ± SE for three subjects and three replicates per subject) compared with their response following exposure to clean air. Asterisks denote significance (p < 0.05).
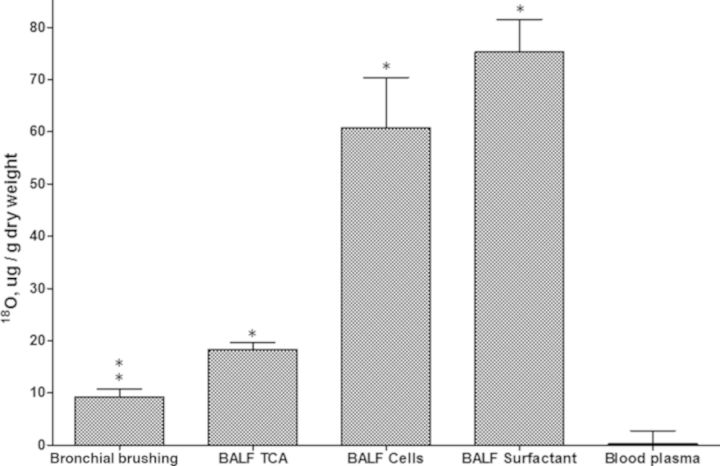
Comparison of 18O in Bronchial Brush Biopsies to Three BALF Fractions
18O incorporation measured after in vivo exposure to 18O3 resulted in higher 18O incorporation into cell and surfactant fractions of BALF than bronchial brush biopsies from the same subjects (Fig. 4). The BALF surfactant incorporated the highest levels of 18O, which had a mean value slightly higher than the BALF cells, fourfold higher than BALF TCA pellet, and about eightfold higher than the mean of the left and right bronchial brush biopsies. A subject-by-subject plot of the relationship between the four tissue pools is shown in Supplementary figure 1.
FIG. 4.
Comparison of 18O incorporation into epithelial cells (shown in Fig. 2) and BALF constituents of the same subjects following exposure to 18O3 (0.3 ppm, 2 h with intermittent exercise). Means ± SE are shown for 10 subjects. All 18O3 exposed tissues had significantly elevated 18O when compared with the natural abundance 18O concentration of blood plasma.
Relationships Between Different In Vivo Sample 18O Measurements
No relationship was observed between the 18O incorporation in the left and right bronchial biopsies. A search for relationships across the 10 human subjects in the measured values for 18O incorporation in different tissue compartments yielded the result that BALF surfactant 18O increased in proportion to BALF cell 18O (R2 = 0.73; see Supplementary fig. 2) but not between other BALF constituents (R2 < 0.5). The concentration of 18O3 measured at the mouthpiece of the subjects (which ranged from 0.21 to 0.33 ppm) did not appear to be correlated with the 18O accumulation in any of the individual tissue or BALF constituents or any combination of the sum of the 18O concentrations in BALF.
DISCUSSION
We achieved a proof-of-concept for obtaining comparable measurements of O3 dose and effect between in vivo exposed epithelial cells and in vitro exposed primary epithelial cells exposed in an air-liquid interface culture. Prior to this, few, if any, equivalent measurements for an air pollutant gas had been identified and compared under both in vivo and in vitro conditions. Problems have included finding equivalent denominators applicable to both types of exposure. For example, measurements of released cytokines or LDH depend on equivalent volumes of extracellular fluid that cannot be controlled equally under in vivo and in vitro conditions. Although the main goal of the study was to compare the dose and response in epithelial cells, some valuable insights were obtained about the relative O3 dose in different cell types. Epithelial cell production of inflammatory mediators appears to be easily measured by gene expression assays, and primary human epithelial cells appear to be a good model that could be expanded in air-liquid interface cultures used in high throughput assays.
This is the first report of 18O incorporation into human bronchial epithelial cells in vivo or into any type of cell exposed to 18O3 in vitro. Our previous studies examined BALF constituents of humans and rats exposed in vivo to 18O3 but did not include epithelial cells (Hatch et al., 1994). It was interesting that both the O3 dose and effect observed in vivo always fell below that observed in the in vitro exposures at the same air concentration of O3. Previous studies that measured O3 air concentrations in the posterior pharynx of exercising human subjects found that fractional removal of O3 in the nose is ∼40% (Gerrity et al., 1994). Thus, scrubbing of O3 in the upper airways might explain the lower dose and effect of O3 in vivo compared with in vitro. Other factors that could have influenced the relative dose and effect between in vivo and in vitro exposures include the following. (1) Brush biopsy of cells in vivo probably removed some underlying cells that would have a lower exposure to O3 than the surface cells, (2) there may have been differences in the competition of the 18O3 reaction with antioxidants that do not form addition products with 18O3 (see below), or (3) a longer (1 h vs. minutes) delay following in vivo exposure compared with in vitro exposure could have resulted in some clearance of 18O or a return to normal gene expression.
It is likely that the bronchial brush biopsy samples measured here gave a low approximation of epithelial cell dose compared with the distal airways that appear to be the main targets for O3 in the human lung. A prior study of resting Rhesus monkeys showed that in vivo exposure (0.4 or 1.0 ppm 18O3 for 2 h) resulted in bronchial cell levels of 18O similar to those observed here for bronchial cells of exercising humans (Plopper et al., 1998): the bronchial cells of monkeys incorporated 4–7 ug 18O/g dry while the human bronchial cells incorporated ∼10 ug 18O/g dry. The smallest airways of the monkey (respiratory bronchioles) had a 2–4-fold higher 18O incorporation than cells of the main bronchus suggesting that human terminal airways would be higher as well. This higher dose deeper in the lung is supported by the 4–8-fold higher 18O incorporation observed here in the BALF constituents. Histopathology data on O3 injury to epithelium and O3 dosimetry uptake models also predict the highest O3 dose and effect in the small terminal airways of all species (Barry et al., 1985; Miller et al, 1978). It would not be possible to biopsy tissues of these small airways in human subjects. The above-mentioned references also demonstrate that although we examined only one dose of 18O3 in the present in vivo study, a dose response behavior should be expected. Taken together, our results suggest that the dose response we observed here in the in vitro exposed epithelial cells covered a reasonable range of doses for human airway epithelial cells exposed in vivo.
In addition to the epithelial cell data, we learned that BALF constituents experience a high dose of 18O3 compared with bronchial epithelial cells. This difference might be due to the larger surface area exposed or biochemical differences in reactivity of the 18O3. We have observed in other studies that low ascorbate is related to high 18O3 levels in BALF (Gunnison and Hatch, 1999; Kari et al., 1997). The lack of ascorbate in the culture media used here would have resulted in a loss of ascorbate from cells during culture (Lane et al., 2013) which would have increased the incorporation of 18O3 in in vitro cells compared with the cells exposed in vivo that are known to be bathed in extracellular ascorbate (Slade et al., 1993; van der Vliet et al., 1999). In an earlier study, we reported a 30–50% lower 18O incorporation into human BALF cells and surfactant pellets than we observed here. This was in spite of the fact that the earlier study involved a higher 18O3 concentration (0.4 ppm, 2 h rather than 0.3 ppm, 2 h) and higher targeted minute ventilation (60 l/min/subject vs. 50 l/min/subject in the present study). One reason for this difference might be that the earlier study measured the 18O3 concentration at a site distant from the breathing zone of the subjects so that surfaces for reaction with 18O3 within the exposure chamber could have reduced the actual concentrations inhaled. In the present study, 18O3 was measured in the breathing zone to better ensure that losses of 18O3 on surfaces would not occur. The in vitro exposures might also be affected by this phenomenon, however, there appeared to be fewer surfaces for reaction with 18O3 in the in vitro chambers than in the in vivo chambers. Another finding is that our earlier study employed dialysis of the BALF supernatant to separate the protein/lipid fraction from the NaCl present in the saline lavage—a necessity because high levels of NaCl can interfere with the 18O assay. The earlier reported 18O levels in the dialyzed BALF high speed supernatant were higher than the present results found in the TCA-precipitated BALF high speed supernatant. It appears that TCA treatment may have dissociated the 18O adduct, or failed to concentrate the same extracellular materials in BALF as did dialysis.
Limitations of the present study include the small number of in vitro replicate exposures as well as of human subjects contributing to the cultures. In addition, we observed significant intra- and inter-subject variations in 18O in cells removed by brush biopsy. Part of this variation may be attributable to the fact that not all the inhaled ozone will react with the surface of airway epithelial cells that are then removed by brush biopsy. Some will react with compounds present in lung lining fluid such as mucin or antioxidants and these compounds may differ in concentration and among people or even in the same person on a day-to-day basis. This is also true to some extent within a subject. It is not known if mucin or antioxidants are distributed evenly throughout the airways. Another factor that may be contributing to variability is that airway cells are not a monolayer and there is a possibility that some of the cells not directly opening into the airway and therefore not receiving a dose of ozone (e.g., basal cells) would be removed during brush scraping to varying degrees from one individual to another or even from different locations within an individual. It is not known if the makeup of cell composition (e.g., % of ciliated cells, mucin secreting cells, clara cells, basal cells, etc.) is homogenous throughout the airway. Finally, some variability was probably due to the limited mass of sample that made it impossible to obtain more than one 18O analysis for each bronchus sampled. Although the BALF cells sampled after in vivo exposure were obtained in a higher sample mass than the tissue mass of bronchial brush biopsies, we believe that the overall contribution of epithelial cells to the inflammatory response would be greater because of a larger surface area exposed to O3 and a higher secretion of inflammatory cytokines in epithelial cells than other cells (Devlin et al., 1994). Future studies should compare the sensitivity to injury of alveolar macrophages and epithelial cells and also determine the contribution of mucus or epithelial lining fluid covering the cells to the resulting dose and effect of O3. Another potential limitation is the possibility that the subjects studied had been exposed previously to ozone and as such might have experienced epigenetic effects that could alter the cell responses following in vitro ozone exposure. No attempt was made to control for this possibility because there are so many other outside influences that could also induce epigenetic effects, and because we presently have no evidence for this type of change in the scenario we have studied here.
In summary, our results suggest that in vitro exposure conditions can be made to reasonably match the in vivo dose of O3 to epithelial cells exposed in vivo and that comparable measures of dose and effect can be obtained. These studies add confidence to the possibility of extrapolating effects observed in vitro to effects that might occur in human airways in vivo.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
United States Environmental Protection Agency intramural funds and a cooperative agreement (CR833463-01) between the U.S. EPA and the Center for Environmental Medicine, Asthma and Lung Biology at the University of North Carolina at Chapel Hill.
Acknowledgments
The authors gratefully acknowledge Maryann Bassett, R.N. and Tracey Montilla, R.N. for their clinical support as well as the members of TRC Environmental Corporation (Raleigh, NC), for their engineering support for the in vivo human 18O3 exposures, and Dr Janice Dye and Dr William Mundy for helpful reviews and suggestions.
REFERENCES
- Barry B. E., Miller F. J., Crapo J. D. Effects of inhalation of 0.12 and 0.25 ppm ozone on proximal alveolar region of juvenile and adult rats. Lab. Invest. 1985;53:692–704. [PubMed] [Google Scholar]
- Devlin R. B., Duncan K. E., Jardim M., Schmitt M. T., Rappold A. G., Diaz-Sanchez D. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation. 2012;126:104–111. doi: 10.1161/CIRCULATIONAHA.112.094359. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Becker S., Madden M. C., McGee M. P., Perez R., Hatch G., House D. E., Koren H. S. Time-dependent changes of inflammatory mediators in the lungs of humans exposed to 0.4 ppm ozone for 2 hr: A comparison of mediators found in bronchoalveolar lavage fluid 1 and 18 hr after exposure. Toxicol. Appl. Pharm. 1996;138:176–185. doi: 10.1006/taap.1996.0111. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., McKinnon K. P., Noah T., Becker S., Koren H. S. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am. J. Physiol. 1994;266(6 Pt 1):L612–L619. doi: 10.1152/ajplung.1994.266.6.L612. [DOI] [PubMed] [Google Scholar]
- Gerrity T. R., McDonnell W. F., House D. E. The relationship between delivered ozone dose and functional responses in humans. Toxicol. Appl. Pharm. 1994;124:275–283. doi: 10.1006/taap.1994.1033. [DOI] [PubMed] [Google Scholar]
- Ghio A. J. B. M., Chall A. N., Levin D. G., Bromberg P. A. Bronchoscopy in healthy volunteers. J. Bronchol. 1998;5:185–194. [Google Scholar]
- Gunnison A. F., Hatch G. E. O3-induced inflammation in prepregnant, pregnant, and lactating rats correlates with O3 dose estimated by 18O. Am. J. Physiol. 1999;276:L332–L340. doi: 10.1152/ajplung.1999.276.2.L332. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., McKee J., Brown J., McDonnell W., Seal E., Soukup J., Slade R., Crissman K., Devlin R. Biomarkers of dose and effect of inhaled ozone in resting versus exercising human subjects: comparison with resting rats. Biomarker Insights. 2013;8:53–67. doi: 10.4137/BMI.S11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Slade R., Harris L. P., McDonnell W. F., Devlin R. B., Koren H. S., Costa D. L., McKee J. Ozone dose and effect in humans and rats—A comparison using 18O labeling and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 1994;150:676–683. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- Kari F., Hatch G., Slade R., Crissman K., Simeonova P. P., Luster M. Dietary restriction mitigates ozone-induced lung inflammation in rats: A role for endogenous antioxidants. Am. J. Respir. Cell Mol. Biol. 1997;17:740–747. doi: 10.1165/ajrcmb.17.6.2844. [DOI] [PubMed] [Google Scholar]
- Lane D. J. R., Chikhani S., Richardson V., Richardson D. R. Transferrin iron uptake is stimulated by ascorbate via an intracellular reductive mechanism. Biochim. Biophys. Acta. 2013;1833:1527–1541. doi: 10.1016/j.bbamcr.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Miller F. J., Menzel D. B, Coffin D. L. Similarity between man and laboratory-animals in regional pulmonarydeposition of ozone. Environ. Res. 1978;17:84–101. doi: 10.1016/0013-9351(78)90064-6. [DOI] [PubMed] [Google Scholar]
- National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, D.C: The National Academies Press; 2007. [Google Scholar]
- Plopper C. G., Hatch G. E., Wong V., Duan X., Weir A. J., Tarkington B. K., Devlin R. B., Becker S., Buckpitt A. R. Relationship of inhaled ozone concentration to acute tracheobronchial epithelial injury, site-specific ozone dose and glutathione depletion in rhesus monkeys. Am. J. Respir. Cell Mol. Biol. 1998;19:387–399. doi: 10.1165/ajrcmb.19.3.3183. [DOI] [PubMed] [Google Scholar]
- Santrock J., Hatch G. E., Slade R., Hayes J. M. Incorporation and disappearance of 18O in lungs from mice exposed to 1 ppm 18O3. Toxicol. Appl. Pharm. 1989;98:75–80. doi: 10.1016/0041-008x(89)90135-x. [DOI] [PubMed] [Google Scholar]
- Slade R., Crissman K., Norwood J., Hatch G. Comparison of antioxidant substances in bronchoalveolar lavage cells and fluid from humans, guinea-pigs, and rats. Exp. Lung Res. 1993;19:469–484. doi: 10.3109/01902149309064358. [DOI] [PubMed] [Google Scholar]
- U. S. E. P. A. Research Triangle Park, NC: 2013. Air quality criteria for ozone and related photochemical oxidants: Integrated Science Assessment. Available at: http://www.epa.gov/ttn/naaqs/standards/ozone/s_o3_2008_isa.html. Accessed June 30, 2014. [Google Scholar]
- van der Vliet A., O'Neill C. A., Cross C. E., Koostra J. M., Volz W. G., Halliwell B., Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. 1999;276:L289–L296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]