Figure 3.
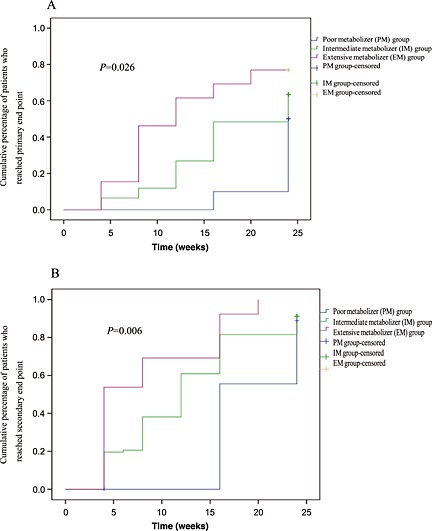
Kaplan–Meier estimates of response rate in different patients. (A) Primary end point patients. (B) Secondary end point of patients. The primary end point was complete remission after 6 months of treatment. Secondary end points included response (defined as partial remission), changes in clinical parameters (including proteinuria, serum albumin, serum creatinine, and serum C3 values), and adverse effects (including leukopenia, infections, gastrointestinal symptoms, amenorrhea, hair loss, liver function disorder, transient increase in serum creatinine level, etc)
