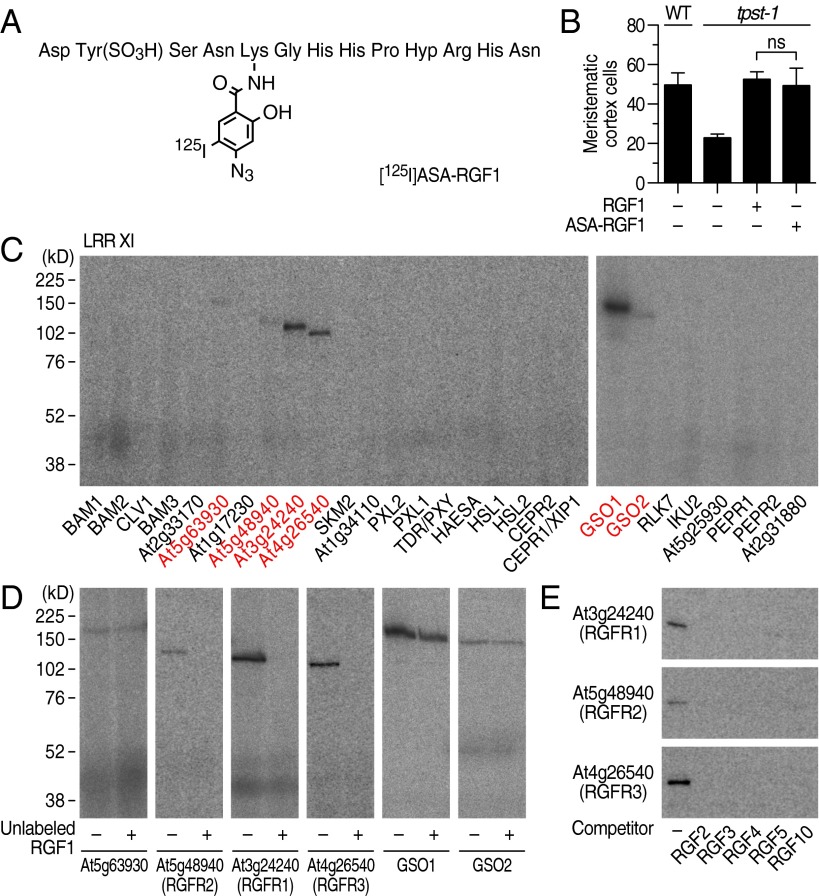
Fig. 1.
Identification of three RKs that specifically interact with RGF by photoaffinity labeling. (A) Structure of [125I]ASA-RGF1. (B) Biological activity of ASA-RGF1 determined by measuring meristematic cell number in the tpst-1 mutant at 100 nM. Data represent mean values ± SD (n = 10–16; ns, not significant; paired t test). (C) Exhaustive photoaffinity labeling using [125I]ASA-RGF1 against membrane fractions derived from individual RK expression lines. (D) Competitive displacement of [125I]ASA-RGF1 binding by 300-fold excess unlabeled RGF1. (E) Competitive displacement of [125I]ASA-RGF1 binding by 300-fold excess of other unlabeled RGF members.