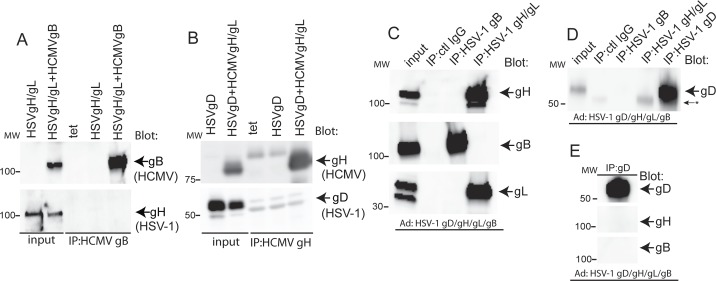
Fig 4. HSV-1 glycoproteins do not interact with HCMV glycoproteins.
(A) ARPE-19 cells were transduced with Ad vectors expressing Ad-tet-trans alone (tet, as a negative control), HSV-1 gH/gL, or HSV-1 gH/gL along with HCMV gB (as indicated along the top of the panel). HCMV gB was IP’d from cell lysates with MAb 15H7 then blots probed with HCMV gB polyclonal serum (upper panel) or anti-HSV-1 gH polyclonal serum R137 (lower panel). (B) ARPE-19 cells were transduced with Ad vectors expressing HSV-1 gD alone or with HSV-1 gD and HCMV gH/gL or with Ad-tet-trans (tet, a negative control) then lysates IP’d with HCMV gH/gL-specific MAb 14-4b. Proteins were analyzed by SDS-PAGE under reducing conditions and western blots were probed with rabbit anti-HSV-1 gD polyclonal serum (R45) or anti-HCMV gH MAb AP86. (C) ARPE-19 cells were transduced with Ad vectors expressing HSV-1 gB, gH/gL, gD, and nectin-1. After 24 hrs proteins were IP’d from cell lysates with anti-HSV-1 gB MAb SS10, anti-HSV-1 gH MAb 53S, or irrelevant control MAb (15H7). The IP’d proteins were separated by SDS-PAGE under reducing conditions, transferred to membranes, and blotted with polyclonal rabbit serum R137 or R67 against HSV-1 gH/gL and gB, respectively. (D) Proteins were IP’d from ARPE-19 lysates with anti-HSV-1 gB MAb SS10, anti-HSV-1 gH MAb 53S, anti-HSV-1 gD MAb DL6, or irrelevant control MAb (15H7). The proteins were separated by SDS-PAGE under reducing conditions, transferred to membranes and blotted with rabbit anti-HSV-1 gD polyclonal serum (R45). The arrow with asterisk indicates cross reactivity of R45 with IgG heavy chain. (E) Proteins were IP’d from cell lysates, separated by SDS-PAGE and transferred to membranes as described above and then probed with rabbit polyclonal serum R45, R137, or R67 against HSV-1 gD, gH/gL, and gB, respectively. Input represents 5% of the total amount of lysate used for the IPs. Molecular mass (MW) markers are indicated on the left.