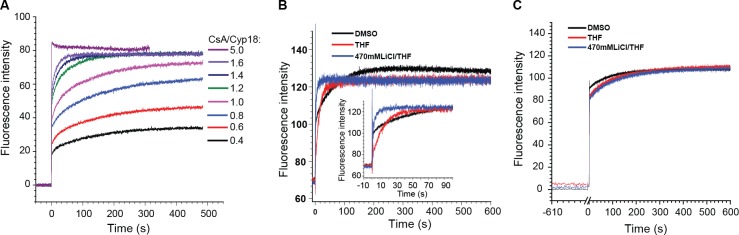
Fig 1. Conformational heterogeneity of CsA in Cyp18 binding.
(A) Fluorescence intensity time courses of Cyp18 (final concentration of 500 nM) upon the addition of various concentrations of CsA using stopped flow. The graph is plotted after subtraction of the initial fluorescence intensity of free Cyp18. Results shown are the average of two independent experiments. The values shown in the inset represent the different ratios of CsA to Cyp18. (B) Fluorescence intensity time courses of Cyp18 upon the addition of CsA dissolved in DMSO (Black), THF (Red) and LiCl/THF (Blue) under continuous stirring. The graph is plotted after subtraction of the reference curve. Results shown are the average of two independent experiments. The inserted graph represents the scale from -10 s to 100 s. (C) Fluorescence intensity time course of Cyp18 upon binding to CsA after incubation in HEPES buffer for 10min (black, red and blue curves are CsA dissolved in DMSO, THF and LiCl/THF before adding to HEPES buffer, respectively). The graph is plotted after subtraction of the reference curve. Results shown are the average of two independent experiments.