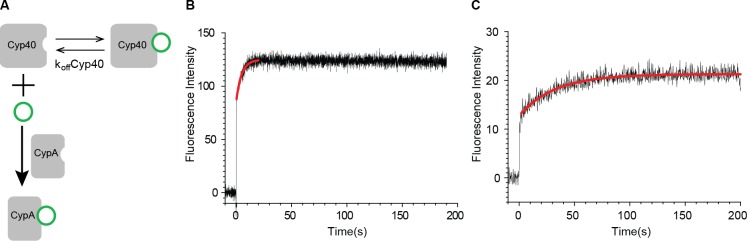
Fig 3. Probing the transient structure of CsA during dissociation from cyclophilin.
(A) Scheme for measuring the transient structure of CsA during dissociation from Cyp40. (B) Fluorescence intensity time course of Cyp18 upon binding to CsA during its dissociation from the Cyp40/CsA complex. The curve was plotted after subtraction of the initial fluorescence intensity of Cyp40. One equivalent of Cyp18 was injected through stopped flow. The time course is fitted to a first-order reaction (k = 0.205 s-1). (C) Fluorescence intensity time course of Cyp18 upon binding to the dissociated CsA from Cyp40. The Cyp40/CsA complex was incubated with a CsA resin to remove the dissociated Cyp40 in solution. After incubating the supernatant at room temperature for half hour, Cyp18 was added and the fluorescent time course was recorded. The kobs value of 0.03067 s-1 of the second slow phase is 7 times slower than the single phase kinetics in (B).