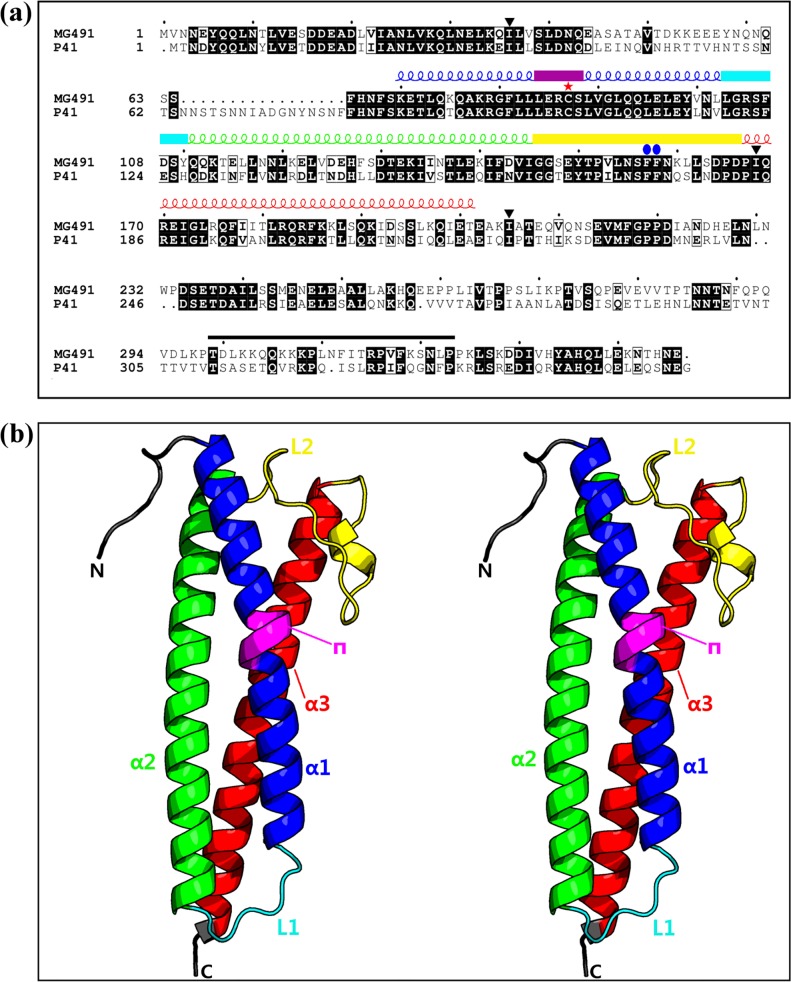
Fig 1. Overall structure of the MG491 monomer.
(a) Sequence alignment of the M. genitalium MG491 protein with its M. pneumoniae homolog, P41. The secondary structure elements found for MG491, as defined with the Dictionary of Secondary Structure of Proteins (DSSP) algorithm [26,27], are reported above the sequence alignment and are colored as: helix α1, α2, α3 and π respectively in blue, green, red and magenta, loop L1 in cyan and loop L2 in yellow. Black solid down triangles, red asterisk and blue solid oblong dots are for residues which have been mutated into methionines (Ile36, Ile168 and Ile 205), serine (Cys87) and alanines (Phe157 and Phe158), respectively. The protein region that interacts with the terminal organelle protein MG200 is indicated with a black bar. Black boxes define conserved regions between MG491 and P41. (b) Stereoview showing the overall structure of a MG491 subunit (PDB entry code 4XNG). The protein backbone is colored according to the color-scheme in (a).