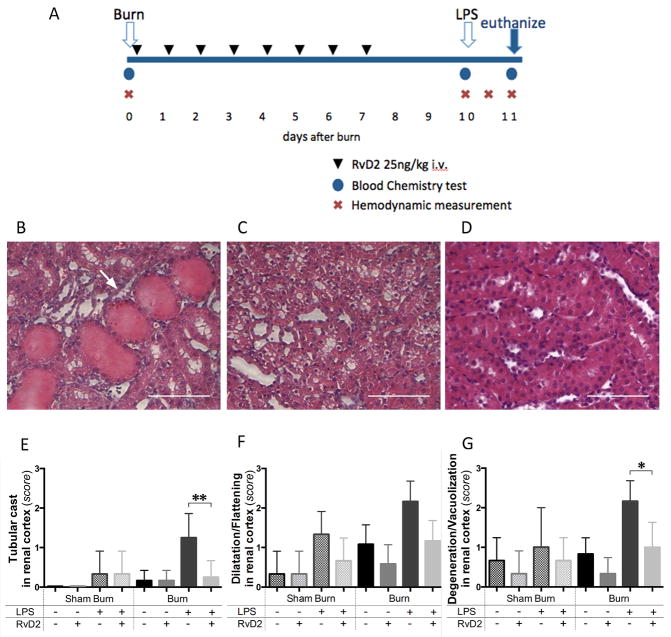
Figure 1. Timeline of the burn injuries, treatment, and renal cortex injury.
A. Blood samples were collected before the burn procedure (day 0), before injecting LPS (day 10 pb), and 24 hours after LPS (day 11 pb). Hemodynamic measurements were also performed at the same time points. One additional measurement was performed at 12 hours after LPS injection. For animals receiving treatment, 25 ng/kg of RvD2 were administered first at 2 hours after burn injury (day 0) and continued daily until day 7 after burn. B, C. Representative section of renal cortex at day 11 pb, in burn and LPS, untreated group. Tubular injuries were observed, including tubular cast formation (arrows), and ballooning degeneration/vacuolization of renal proximal tubular cells (H&E, ×400, scale bar 50 μm). D. Light microscopic image at day 11 pb in burn and LPS, RvD2 treated group, indicated that tubular injury was reduced by RvD2 treatment in renal cortex (H&E, ×400, scale bar 50 μm). E – G. Quantitative analysis of pathological changes in the renal cortex (N=3 rats total, N=2–3 experiments for each sham burn group; N=6 rats total, N=3–4 experiments for each burn group). We employed semi-quantitative scores (from 0 (normal) to 3 (severe)), as previously described (17)). We assessed the presence of tubular casts, tubular dilatation/flattening, and tubular degeneration/vacuolization in the renal cortex. In all comparisons, RvD2 treatment reduced the pathological changes of tubular cells in renal cortex (Burn/LPS vs. Burn/LPS/RvD2; **p≤0.01, ns, and *p≤0.05, respectively).