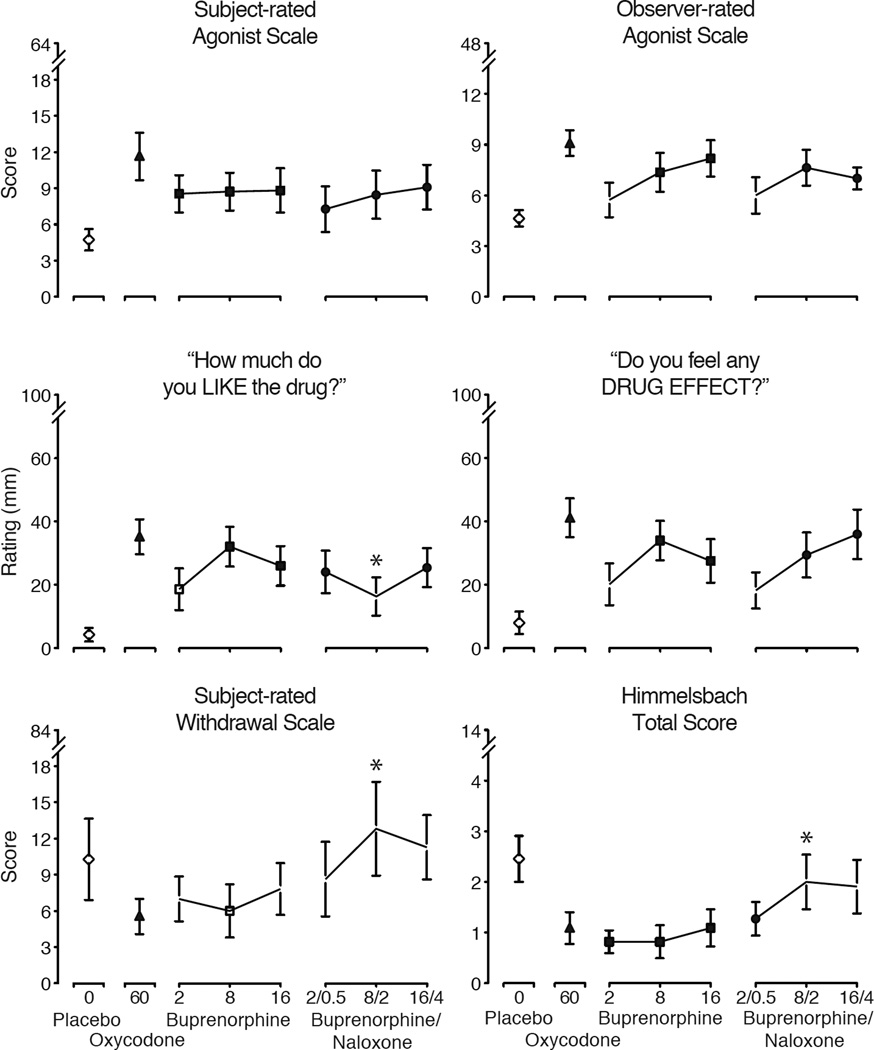
Figure 2.
Mean peak data (n=11; ± 1 SEM) are shown for six measures reflecting opioid agonist effects, abuse potential and opioid withdrawal from the subjects and observers after intranasal administration of placebo (diamond), oxycodone (triangle), BUP alone (square) and BUP/NX (circles). For all measures, there were statistically significant main effects of dose as follows: Subject-rated Agonist Scale (upper left; F [7, 70] = 5.43; p<.0001), Observer-rated Agonist Scale (upper right; F=3.86; p=.001), the VAS “How much do you LIKE the drug?” (center left; F=3.48; p=.003), the VAS “Do you feel any DRUG EFFECT?” (center right; F=3.81; p=.002), Subject-rated Withdrawal Scale (lower left; F=2.45; p=.027) and the observer-rated Himmelsbach Scale (lower right; F=3.41; p=.003). Filled symbols indicate significant post-hoc differences between active doses and placebo; asterisks (*) indicate significant difference between matched doses of BUP vs. BUP/NX (Fisher’s LSD test, p<.05).