Figure 4. Spatiotemporal association of mutations in pediatric high-grade glioma.
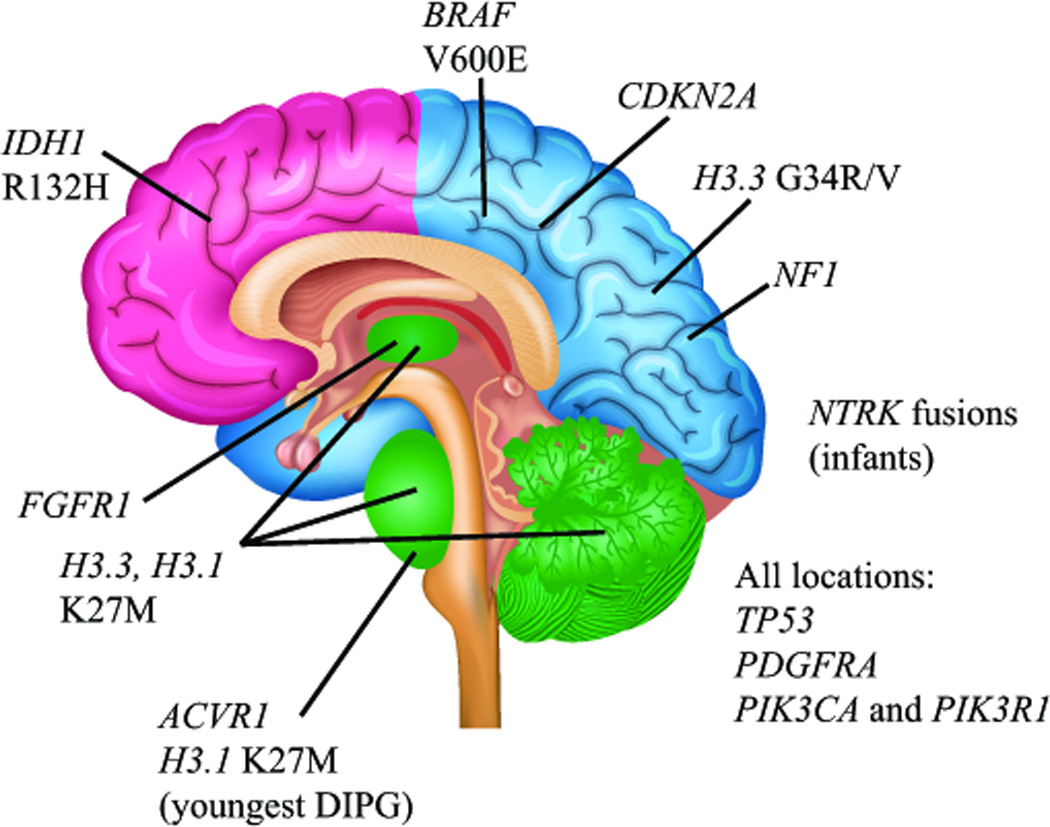
Clear associations between specific mutations and age- and location-dependent subgroups of pediatric HGG indicate a strong connection between brain development and gliomagenesis in children. Histone H3 K27M mutations are found predominantly in DIPGs, and other midline HGGs including thalamic and cerebellar (green), with the majority of mutations occurring in the H3.3 isoform. The youngest DIPG patients show a predominance of ACVR1 mutations and K27M mutations in the H3.1 isoform. Histone H3.3 G34R/V mutations are predominantly found in cortical HGGs of late adolescence, most commonly those arising in the parietal, occipital, or temporal lobes (blue). In contrast, frontal lobe HGGs of late adolescence and young adults are more commonly associated with IDH1 R132H mutation (pink). BRAFV600E mutations and CDKN2A homozygous deletions are found in non-brainstem HGGs and not DIPGs, and NF1 mutations are more frequent in cortical HGGs than DIPG. NTRK fusion genes have been reported in non-brainstem HGGs as well as DIPGs, but appear to occur at higher frequency in HGGs arising outside the brainstem in children less than 3 years of age. FGFR1 mutations are found predominantly in thalamic HGGs. Mutations in TP53, PDGFRA, PIK3CA and PIK3R1 are found in HGGs from all locations.
