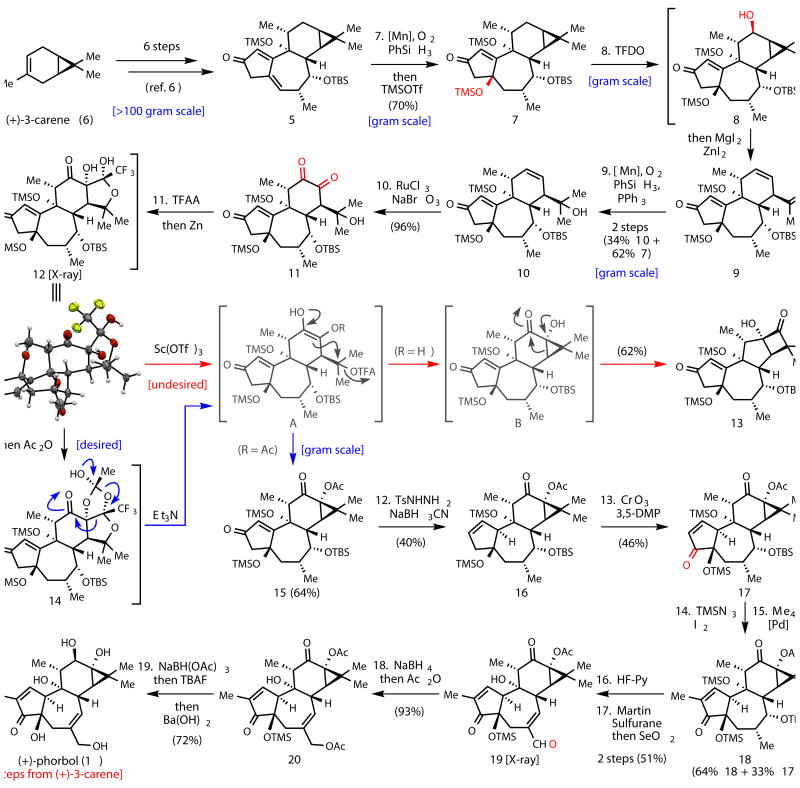
Figure 2.
19-step total synthesis of 1. Reagents and conditions: 7. Mn(acac)2, PhSiH3, O2, EtOH, then TMSOTf, Et3N, CH2Cl2, 0 °C, 70%. 8. TFDO, CH2Cl2, 0 °C, then ZnI2, MgI2, Et2O. 9. Mn(acac)2, PhSiH3, O2, PPh3, EtOH, 2 steps (34% 10 + 62% 7). 10. RuCl3, NaBrO3, NaHCO3, EtOAc, CH3CN, H2O, 96%. 11. TFAA, DMAP, CH2Cl2, 0 °C, then Zn, AcOH, CH2Cl2, then Ac2O, DMAP, CH2Cl2, 0 °C, then Et3N, DMF, 60 °C, 64%. 12. TsNHNH2, MeOH, reflux, then NaBH3CN, AcOH, reflux, 40%. 13. CrO3, 3,5-DMP, CH2Cl2, 0 °C to rt, 46%. 14. TMSN3, DCE, 70 °C, then I2, DCE, pyridine, 70 °C. 15. Me4Sn, PdCl2(PhCN)2, AsPh3, CuI, NMP, 80 °C, 2 steps (64% 18 + 33% 17). 16. HF·Pyridine, THF, 0 °C. 17. Martin Sulfurane, DCE, 60 °C, then SeO2, benzene, 80 °C, 2 steps 51%. 18. NaBH4, MeOH, -40 °C, then Ac2O, DMAP, CH2Cl2, 93%. 19. NaBH(OAc)3, benzene, reflux, then TBAF, THF, 0 °C, then Ba(OH)2, MeOH, 72%.