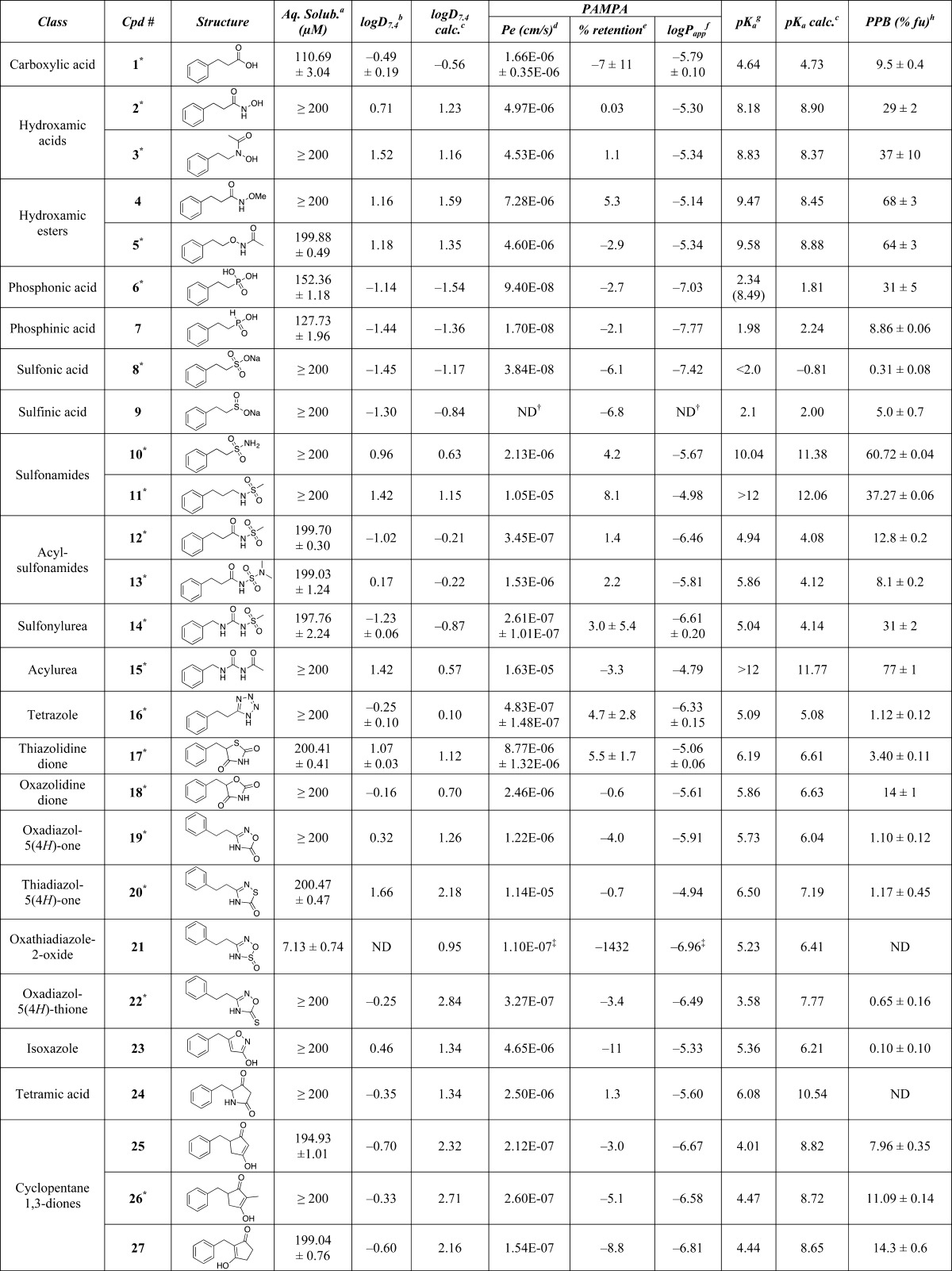
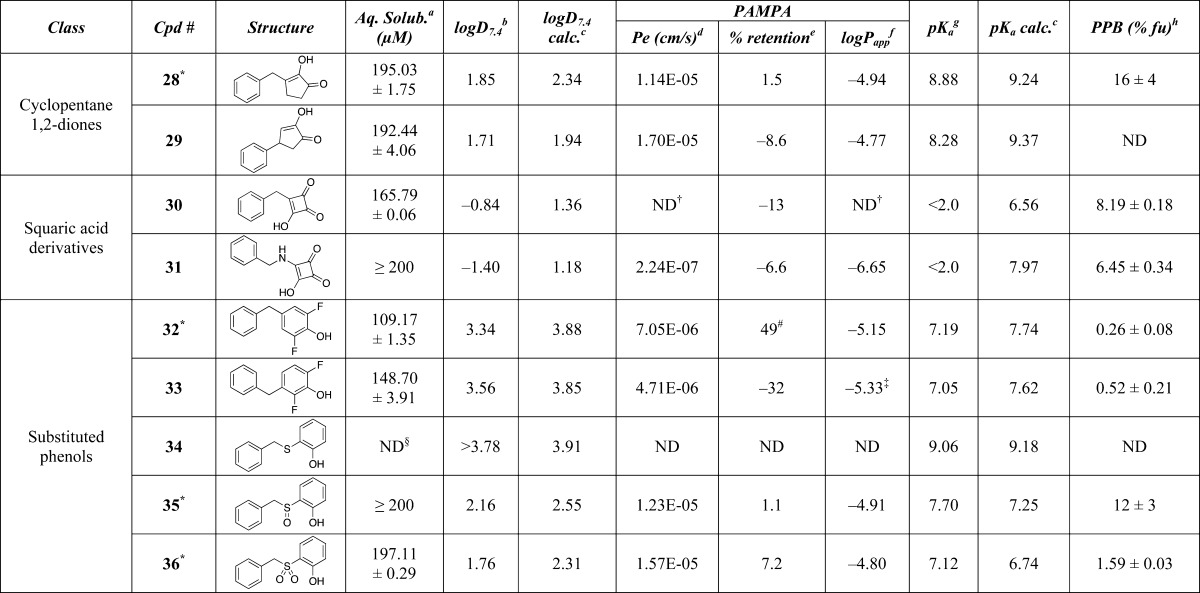
Table 1. Calculated and Experimental Properties of Test Compounds.
Kinetic solubility in aqueous phosphate buffer (pH 7.4) determined by LC/MS after 24 h of incubation (experiment run by Analyza).
Distribution coefficient between n-octanol and aqueous buffer (pH 7.4) determined by LC/MS (experiment run by WuXi AppTech).
Calculated values using ChemAxon.10
Effective permeability (PAMPA assay run by Analyza).
Membrane retention.
Log of the apparent permeability coefficient.
pKa values determined by capillary electrophoresis; for diprotic compounds, the second equivalence point is indicated in brackets (experiment run by Analyza).
Plasma protein binding, fraction unbound (fu) determined by equilibrium dialysis.
The X-ray crystal structure revealing the H-bond pattern is shown in the Supporting Information.
The permeability value could not be calculated, as the concentration of test compound in the acceptor plate was below the limit of quantitation (<LOQ).
Compound precipitated in donor plate.
Compound appeared to exhibit high nonspecific binding.
Compound appeared to be unstable during the 24 h incubation time of the assay; ND = not determined.