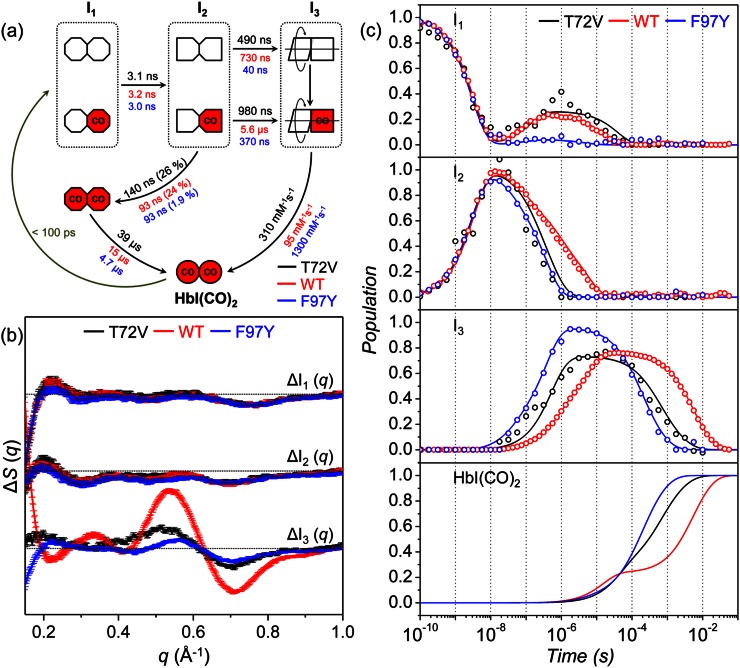
FIG. 3.
(a) Kinetic model for T72V HbI. Time constants for T72V, wild type, and F97Y are represented in black, red, and blue, respectively. The red (with “CO”) and white symbols represent ligated and photolyzed subunits, respectively. To indicate the change in tertiary structure with the progress of structural transition, we represented the subunits of each intermediate with symbols of different shapes. To indicate the change in quaternary structure in the transition from I2 to I3, we described the two subunits of I3 rotating with respect to each other. Two red octagons represent a ligated form of I1, which is formed by geminate recombination of CO with I2 and is structurally indistinguishable from the photolyzed forms of I1. (b) Species-associated difference scattering curves for the three intermediates of T72V (black), wild-type (red), and F97Y (blue) HbI. (c) Population changes of the three intermediates and HbI(CO)2 for T72V (black lines), wild-type (red lines), and F97Y (blue lines) HbI obtained by kinetic analyses. The open circles represent the optimized populations obtained by fitting the experimental difference scattering curves with the species-associated difference scattering curves of the three intermediates shown in (b). We note that the population of I1 rises back at ∼10 ns because geminate recombination of CO with the I2 intermediate leads to the formation of a ligated form of I1 (indicated by two red octagons in (a), which is structurally indistinguishable from the photolyzed forms of I1.