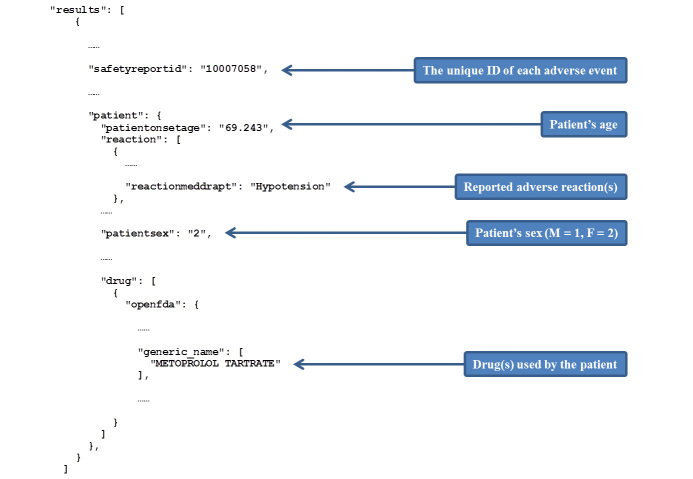
Figure 1.
Co-occurrence of drug and adverse reaction. An adverse event with unique identifier (ID) can be queried in the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), which returns results as JavaScript Object Notation by default. In this illustrated adverse event (with partial content displayed), the use of metoprolol and the incidence of hypotension reaction are reported simultaneously, which is defined as co-occurrence.