Figure 7.
Rex1 and Zscan4 Correlate with the Cell-Cycle Length Under Different Mechanisms
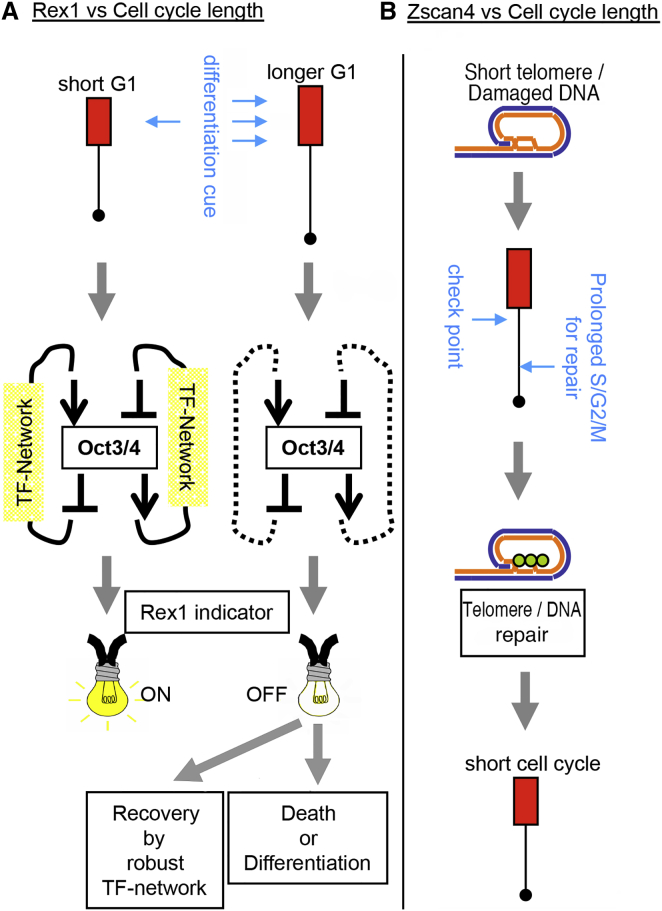
(A) ESCs are continuously exposed to differentiation factors. For example, a very general growth factor, fibroblast growth factor (FGF), is always around ESCs as it is secreted by ESCs themselves (Kunath et al., 2007) and may also be contained in the media if supplemented with serum. Downstream of FGF, MAPK is activated, which suppresses pluripotent factors such as Tbx3 and Nanog (Niwa et al., 2009). But ESCs form a robust transcription factor (TF) network that gradually adjusts the pluripotent status when the balance among the TFs becomes chaotic (Niwa et al., 2009). Since the battle between differentiation and pluripotency occurs at the transcription level, the conditions at the G1 phase should be the platform (Pauklin and Vallier, 2013). Thus, when the G1 phase (red box in this figure) is long, there are higher chances for the differentiation cues to invade, which then disturbs the TF network so the expression level of the master gene Oct3/4 becomes altered and, as a consequence, the indicator Rex1 turns off. Although Rex1 is off, this should be distinguished from complete differentiation, because even at the longer cell cycles discussed here, the G1 phase remains in a typical ESC-like proportion (Figure 1D), and both the cell-cycle length (Figures 1A, 2C, and S5) and Rex1 expression (Figure S3A) are reversible. In conventional culture (i.e., in serum-containing medium supplemented with LIF), ESCs are probably fluctuating between the two statuses illustrated on the left and right in (A). At longer cell cycles, the chances of invasion by differentiation cues can be higher, resulting in negative correlation of Rex1 and the cell-cycle length (Figure S3B).
(B) In the event of telomere shortening beyond the threshold or DNA damage, DNA replication is paused until the repair is complete, which results in a prolonged S/G2/M phase. This probably underlies the activation of Zscan4 at longer cell cycles (Figures 4A and 4B).