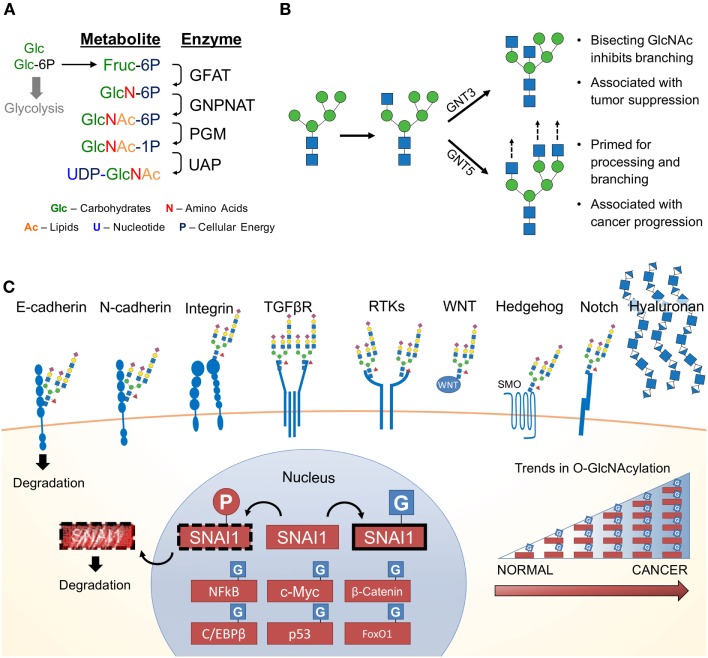
Figure 2.
The hexosamine biosynthetic pathway (HBP) and glycosylated EMT targets. (A) First, the rate limiting enzyme of the HBP, glutamine:fructose-6-phosphate transaminase (GFAT), uses glutamine (Gln) as an amine donor to convert Fru-6P into glucosamine-6-P (GlcN-6P). Second, glucosamine-phosphate N-acetyltransferase (GNPNAT) N-acetylates GlcN-6P in an acetyl-CoA-mediated reaction to form N-acetylglucosamine-6-P (GlcNAc-6P). Third, phosphoglucomutase (PGM) isomerizes GlcNAc-6P to the highly active GlcNAc-1P. The final step is catalyzed by UDP–N-acetylglucosamine pyrophosphorylase (UAP1) and charges GlcNAc-1P with UDP to form uridine-5′-diphosphate-N-acetylglucosamine (UDP–GlcNAc). (B) UDP–GlcNAc (depicted as a blue square) is essential for N-glycosylation processing and elongation. One critical pivot point includes the branching of complex N-glycans. Inhibiting this process with a bisecting GlcNAc is associated with tumor suppressive phenotypes. In contrast, cancers have aberrant expression of glycosyltransferases responsible for branching and elongating complex N-glycans. (C) Many of the proteins commonly associated with promoting EMT are modified by glycans containing GlcNAc and are found on the cell surface. Hyaluronan, a glycosaminoglycan, is also found extracellularly and is a polymer of glucuronic acid and N-acetylglucosamine. Many nuclear, cytoplasmic and mitochondrial proteins are modified by monosaccharides of O-linked N-acetylglucosamine (O-GlcNAc), including many transcription factors, which appear to be stabilized by glycosylation (63). Numerous studies have identified various cancers with elevated levels of pan-O-GlcNAcylation (64).